

Cholbam 胆酸胶囊




| 通用中文 | 胆酸胶囊 | 通用外文 | cholic acid |
| 品牌中文 | 品牌外文 | Cholbam | |
| 其他名称 | |||
| 公司 | Asklepion(Asklepion) | 产地 | 美国(USA) |
| 含量 | 250mg | 包装 | 90粒/盒 |
| 剂型给药 | 胶囊 口服 | 储存 | 室温 |
| 适用范围 | ⑴胆酸合成疾病由于单酶缺陷(SEDs)的治疗。 ⑵过氧化物酶体病(PDs)的辅助性治疗包括Zellweger谱疾病在肝病表现,脂肪泻或来自脂溶性维生素吸收减低合并症患者。 | ||
| 通用中文 | 胆酸胶囊 |
| 通用外文 | cholic acid |
| 品牌中文 | |
| 品牌外文 | Cholbam |
| 其他名称 | |
| 公司 | Asklepion(Asklepion) |
| 产地 | 美国(USA) |
| 含量 | 250mg |
| 包装 | 90粒/盒 |
| 剂型给药 | 胶囊 口服 |
| 储存 | 室温 |
| 适用范围 | ⑴胆酸合成疾病由于单酶缺陷(SEDs)的治疗。 ⑵过氧化物酶体病(PDs)的辅助性治疗包括Zellweger谱疾病在肝病表现,脂肪泻或来自脂溶性维生素吸收减低合并症患者。 |
美国FDA批准Cholbam(cholic acid,中文译名:胆酸)胶囊上市,治疗一种罕见的,因单一合成酶缺陷引起的胆汁酸合成障碍(bile acid synthesis disorders)和包括Zellweger综合征在内的过氧化物酶障碍(peroxisomal disorders)。这是美国FDA批准的首款治疗这类儿童或成人患者的药物。
胆汁酸合成障碍(bile acid synthesis disorders)是一种极为罕见的,遗传性的代谢疾病。胆汁酸合成障碍因胆固醇代谢过程中的一个或多个环节障碍引起。胆固醇代谢至胆汁酸需要17步化学转化以及16个酶参与。有证据表明缺少其中11个酶当中的任何一个都引起相关疾病。比如修饰胆固醇核环结构部分酶的缺陷导致进行性胆汁淤积性肝病,临床表现血清肝酶升高、高结合胆红素血症及脂溶性维生素吸收不良,胆固醇侧链修饰酶的缺陷则常导致神经系统功能紊乱。如果不及时治疗,胆汁酸合成障碍会导致儿童残疾甚至危及生命。
批准日期:2015年3月22日 公司:Asklepion
CHOLBAM(胆酸[Cholic Acid])胶囊,为口服使用
美国初次批准: 2015
作用机制
胆酸是在肝脏中从胆固醇合成的主要胆酸。由于单酶缺陷(SEDs)在生物合成通路在胆酸合成疾病,和在过氧化物酶体病(PDs)包括Zellweger谱疾病,主要胆酸的缺乏导致中间体胆酸的非调节积蓄和胆汁淤积。胆酸s 促进脂肪消化和通过形成混合胶束吸收,和促进在小肠中脂溶性维生素的吸收。
内源性胆酸包括胆酸增强胆汁流和提供胆酸合成的生理学反馈抑制作用。胆酸的作用机制尚未完全确定;但是,已知胆酸及其结合物是核受体,法尼醇X受体(FXR)的内源性配体。FXR 调节酶和转运蛋白涉及胆酸合成和肠肝循环中维持胆酸正常生理条件下稳态平衡。
适应证和用途
CHOLBAM是一种胆酸适用为:
⑴胆酸合成疾病由于单酶缺陷(SEDs)的治疗。
⑵过氧化物酶体病(PDs)的辅助性治疗包括Zellweger谱疾病在肝病表现,脂肪泻或来自脂溶性维生素吸收减低合并症患者。
使用限制: 尚未确定CHOLBAM对由于SEDs或过氧化物酶体病(PDs)包括Zellweger谱疾病的胆酸合成疾病感外表现的安全性和有效性。
剂量和给药方法
⑴在儿童患者和成年中,推荐剂量是10至15 mg/kg每天1次或分两次服用。见处方资料对基于体重给药表。
⑵在有同时家族性高甘油三脂血症患者中推荐剂量是11至17 mg/kg每天1次或分两次服用和根据临床反应调整。
⑶对头3个月每个月,对下9个月每3个月,下三年期间每6个月和其后每年,监视AST,ALT,GGT,碱性磷酸酶,胆红素和INR。给予有效维持肝功能最低剂量。
⑷如开始治疗的3个月内肝功能没有改善,如发生完全胆梗阻,或如有持续肝功能或胆汁淤积临床和实验室恶化的指示终止CHOLBAM;继续对肝功能监视和当参数返回至基线考虑较低剂量重新开始。
给药指导:
⑴与食物服用。
⑵不要压碎或咀嚼胶囊。对不能吞咽胶囊患者,可开开胶囊和内容物与饮料/食物混合。
剂型和规格
胶囊: 50mg,250mg
禁忌证
无
警告和注意事项
肝受损加重: 监视肝功能和如当用治疗时肝功能恶化终止CHOLBAM.
不良反应
最常见不良反应(≥1%)是腹泻,反流性食道炎,全身乏力,黄疸,皮肤病变,恶心,腹痛,肠息肉,泌尿道感染,和周围神经病变。
药物相互作用
⑴胆汁盐外排泵(BSEP)抑制剂(如,环孢霉素[cyclosporine]): 避免同时使用;如需要同时使用,监视血清转氨酶和胆红素.
⑵胆酸树脂和铝基抗酸剂: 胆酸结合树脂或铝基抗酸剂至少1小时前或4至6小时(或间隔尽可能大)服用CHOLBAM。
如何供应/贮存和处置
50mg胶囊
可得到CHOLBAM胶囊作为两-粒明胶胶囊有瑞典橙帽印有“50mg”和瑞典橙体印有“ASK001”.胶囊含白或灰白粉和在以下瓶供应:
● 90粒胶囊(NDC 43472-001-02)
250mg胶囊
可得到CHOLBAM胶囊为两-粒明胶胶囊有白帽印有“250mg”和白体印有“ASK002”。胶囊含白或灰白粉和以下瓶供应:
● 90粒胶囊(NDC 43472-002-02)
贮存和处置
贮存在20–25°C(69-77°F),外出允许15-30°C(59-86°F)间[见USP控制室温]。
美国FDA批准Cholbam(cholic acid,中文译名:胆酸)胶囊上市,治疗一种罕见的,因单一合成酶缺陷引起的胆汁酸合成障碍(bile acid synthesis disorders)和包括Zellweger综合征在内的过氧化物酶障碍(peroxisomal disorders)。这是美国FDA批准的首款治疗这类儿童或成人患者的药物。
胆汁酸合成障碍(bile acid synthesis disorders)是一种极为罕见的,遗传性的代谢疾病。胆汁酸合成障碍因胆固醇代谢过程中的一个或多个环节障碍引起。胆固醇代谢至胆汁酸需要17步化学转化以及16个酶参与。有证据表明缺少其中11个酶当中的任何一个都引起相关疾病。比如修饰胆固醇核环结构部分酶的缺陷导致进行性胆汁淤积性肝病,临床表现血清肝酶升高、高结合胆红素血症及脂溶性维生素吸收不良,胆固醇侧链修饰酶的缺陷则常导致神经系统功能紊乱。如果不及时治疗,胆汁酸合成障碍会导致儿童残疾甚至危及生命。
批准日期:2015年3月22日 公司:Asklepion
CHOLBAM(胆酸[Cholic Acid])胶囊,为口服使用
美国初次批准: 2015
作用机制
胆酸是在肝脏中从胆固醇合成的主要胆酸。由于单酶缺陷(SEDs)在生物合成通路在胆酸合成疾病,和在过氧化物酶体病(PDs)包括Zellweger谱疾病,主要胆酸的缺乏导致中间体胆酸的非调节积蓄和胆汁淤积。胆酸s 促进脂肪消化和通过形成混合胶束吸收,和促进在小肠中脂溶性维生素的吸收。
内源性胆酸包括胆酸增强胆汁流和提供胆酸合成的生理学反馈抑制作用。胆酸的作用机制尚未完全确定;但是,已知胆酸及其结合物是核受体,法尼醇X受体(FXR)的内源性配体。FXR 调节酶和转运蛋白涉及胆酸合成和肠肝循环中维持胆酸正常生理条件下稳态平衡。
适应证和用途
CHOLBAM是一种胆酸适用为:
⑴胆酸合成疾病由于单酶缺陷(SEDs)的治疗。
⑵过氧化物酶体病(PDs)的辅助性治疗包括Zellweger谱疾病在肝病表现,脂肪泻或来自脂溶性维生素吸收减低合并症患者。
使用限制: 尚未确定CHOLBAM对由于SEDs或过氧化物酶体病(PDs)包括Zellweger谱疾病的胆酸合成疾病感外表现的安全性和有效性。
剂量和给药方法
⑴在儿童患者和成年中,推荐剂量是10至15 mg/kg每天1次或分两次服用。见处方资料对基于体重给药表。
⑵在有同时家族性高甘油三脂血症患者中推荐剂量是11至17 mg/kg每天1次或分两次服用和根据临床反应调整。
⑶对头3个月每个月,对下9个月每3个月,下三年期间每6个月和其后每年,监视AST,ALT,GGT,碱性磷酸酶,胆红素和INR。给予有效维持肝功能最低剂量。
⑷如开始治疗的3个月内肝功能没有改善,如发生完全胆梗阻,或如有持续肝功能或胆汁淤积临床和实验室恶化的指示终止CHOLBAM;继续对肝功能监视和当参数返回至基线考虑较低剂量重新开始。
给药指导:
⑴与食物服用。
⑵不要压碎或咀嚼胶囊。对不能吞咽胶囊患者,可开开胶囊和内容物与饮料/食物混合。
剂型和规格
胶囊: 50mg,250mg
禁忌证
无
警告和注意事项
肝受损加重: 监视肝功能和如当用治疗时肝功能恶化终止CHOLBAM.
不良反应
最常见不良反应(≥1%)是腹泻,反流性食道炎,全身乏力,黄疸,皮肤病变,恶心,腹痛,肠息肉,泌尿道感染,和周围神经病变。
药物相互作用
⑴胆汁盐外排泵(BSEP)抑制剂(如,环孢霉素[cyclosporine]): 避免同时使用;如需要同时使用,监视血清转氨酶和胆红素.
⑵胆酸树脂和铝基抗酸剂: 胆酸结合树脂或铝基抗酸剂至少1小时前或4至6小时(或间隔尽可能大)服用CHOLBAM。
如何供应/贮存和处置
50mg胶囊
可得到CHOLBAM胶囊作为两-粒明胶胶囊有瑞典橙帽印有“50mg”和瑞典橙体印有“ASK001”.胶囊含白或灰白粉和在以下瓶供应:
● 90粒胶囊(NDC 43472-001-02)
250mg胶囊
可得到CHOLBAM胶囊为两-粒明胶胶囊有白帽印有“250mg”和白体印有“ASK002”。胶囊含白或灰白粉和以下瓶供应:
● 90粒胶囊(NDC 43472-002-02)
贮存和处置
贮存在20–25°C(69-77°F),外出允许15-30°C(59-86°F)间[见USP控制室温]。
Generic Name: cholic acid
Dosage Form: capsule
Medically reviewed by Drugs.com. Last updated on Jul 1, 2019.
OverviewSide EffectsDosageProfessionalInteractionsMoreCholbam is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects (SEDs) [see Clinical Trials (14.1)].
Peroxisomal Disorders Including Zellweger Spectrum DisordersCholbam is indicated for adjunctive treatment of peroxisomal disorders (PDs) including Zellweger spectrum disorders in patients who exhibit manifestations of liver disease, steatorrhea or complications from decreased fat soluble vitamin absorption [see Clinical Trials (14.2)].
Limitation of UseThe safety and effectiveness of Cholbam on extrahepatic manifestations of bile acid synthesis disorders due to SEDs or PDs including Zellweger spectrum disorders have not been established.
Cholbam Dosage and AdministrationDosage Regimen for Bile Acid Synthesis Disorders due to Single Enzyme Defects and Peroxisomal Disorders including Zellweger Spectrum DisordersThe recommended dosage of Cholbam is 10 to 15 mg/kg administered orally once daily, or in two divided doses, in pediatric patients and in adults.
Tables 1 and 2 show the number of capsules that should be administered daily to approximate a 10 mg/kg/day and 15 mg/kg/day dosage, respectively, using the available 50 mg and 250 mg capsules alone or in combination.
| 10 mg/kg/day Dosage | ||
|---|---|---|
| Body Weight (kg) | Number of 50 mg capsules | Number of 250 mg capsules |
| 4 to 6 | 1 | 0 |
| 7 to 10 | 2 | 0 |
| 11 to 15 | 3 | 0 |
| 16 to 20 | 4 | 0 |
| 21 to 25 | 0 | 1 |
| 26 to 30 | 1 | 1 |
| 31 to 35 | 2 | 1 |
| 36 to 40 | 3 | 1 |
| 41 to 45 | 4 | 1 |
| 46 to 50 | 0 | 2 |
| 51 to 55 | 1 | 2 |
| 56 to 60 | 2 | 2 |
| 61 to 65 | 3 | 2 |
| 66 to 70 | 4 | 2 |
| 71 to 75 | 0 | 3 |
| 76 to 80 | 1 | 3 |
| 15 mg/kg/day Dosage | ||
|---|---|---|
| Body Weight (kg) | Number of 50 mg capsules | Number of 250 mg capsules |
| 4 to 5 | 1 | 0 |
| 6 to 9 | 2 | 0 |
| 10 to 13 | 3 | 0 |
| 14 to 16 | 4 | 0 |
| 17 to 19 | 0 | 1 |
| 20 to 23 | 1 | 1 |
| 24 to 26 | 2 | 1 |
| 27 to 29 | 3 | 1 |
| 30 to 33 | 4 | 1 |
| 34 to 36 | 0 | 2 |
| 37 to 39 | 1 | 2 |
| 40 to 43 | 2 | 2 |
| 44 to 46 | 3 | 2 |
| 47 to 49 | 4 | 2 |
| 50 to 53 | 0 | 3 |
| 54 to 56 | 1 | 3 |
| 57 to 59 | 2 | 3 |
| 60 to 63 | 3 | 3 |
| 64 to 66 | 4 | 3 |
| 67 to 69 | 0 | 4 |
| 70 to 73 | 1 | 4 |
| 74 to 76 | 2 | 4 |
| 77 to 79 | 3 | 4 |
| 80 | 4 | 4 |
Patients with newly diagnosed, or a family history of, familial hypertriglyceridemia may have poor absorption of Cholbam from the intestine and require a 10% increase in the recommended dosage to account for losses due to malabsorption. The recommended dosage of Cholbam in patients with concomitant familial hypertriglyceridemia is 11 to 17 mg/kg orally once daily, or in two divided doses. Adequacy of the dosage regimen can be determined by monitoring of patients' clinical response including steatorrhea, and laboratory values including transaminases, bilirubin and PT/INR.
Treatment MonitoringTreatment with Cholbam should be initiated and monitored by an experienced hepatologist or pediatric gastroenterologist.
Monitor serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), serum gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), bilirubin and INR every month for the first 3 months, every 3 months for the next 9 months, every 6 months during the subsequent three years and annually thereafter. Monitor more frequently during periods of rapid growth, concomitant disease, and pregnancy. Administer the lowest dose of Cholbam that effectively maintains liver function [see Warnings and Precautions (5.1)] .
Discontinue treatment with Cholbam if liver function does not improve within 3 months of the start of treatment or complete biliary obstruction develops.
Discontinue treatment with Cholbam at any time if there are persistent clinical or laboratory indicators of worsening liver function or cholestasis [see Warnings and Precautions (5.1)] . Concurrent elevations of serum gamma glutamyltransferase (GGT) and serum alanine aminotransferase (ALT) may indicate Cholbam overdose [ see Overdosage (10)]. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
Assessment of serum or urinary bile acid levels using mass spectrometry is used in the diagnosis of bile acid synthesis disorders due to SEDs and PDs including Zellweger spectrum disorders. The utility of bile acid measurements in monitoring the clinical course of patients and in decisions regarding dose adjustment has not been demonstrated.
Administration InstructionsTake Cholbam with food.Take Cholbam at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin or aluminum-based antacid.Do not crush or chew the capsules.For patients unable to swallow the capsules, the capsules can be opened and the contents mixed with either infant formula or expressed breast milk (for younger children), or soft food such as mashed potatoes or apple puree (for older children and adults) in order to mask any unpleasant taste:Hold the capsule over the prepared liquid/food, gently twist open, and allow the contents to fall into the liquid/food.Mix the entire capsule contents with one or two tablespoons (15 mL to 30 mL) of infant formula, expressed breast milk, or soft food such as mashed potatoes or apple puree.Stir for 30 seconds.The capsule contents will remain as fine granules in the milk or food, and will not dissolve.Administer the mixture immediatelyDosage Forms and StrengthsCholbam is available in two capsule strengths.
50 mg capsule: Size number 2 Swedish orange capsule with cap imprinted with "50mg" and body imprinted with "ASK001". The capsules contain a white to off-white powder.250 mg capsule: Size number 0 white capsule with a cap imprinted with "250mg" and body imprinted with "ASK002". The capsules contain a white to off-white powder.ContraindicationsNone.
Warnings and PrecautionsExacerbation of Liver ImpairmentMonitor liver function and discontinue Cholbam in patients who develop worsening of liver function while on treatment. Concurrent elevations of serum gamma glutamyltransferase (GGT), alanine aminotransferase (ALT) may indicate Cholbam overdose. [see Overdosage (10)]. Discontinue treatment with Cholbam at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis.
Evidence of liver impairment was present before treatment with Cholbam in approximately 86% (44/51) of patients with bile acid synthesis disorders due to SEDs and in approximately 50% (14/28) of patients with PDs including Zellweger spectrum disorders. Five of the patients (3 SED and 2 PD) with liver impairment at baseline experienced worsening serum transaminases, elevated bilirubin values, or worsening cholestasis on liver biopsy following treatment. An additional 5 patients (2 SED and 3 PD) who did not have baseline cholestasis experienced an exacerbation of their liver disease while on treatment. Exacerbation of liver impairment by Cholbam in these patients cannot be ruled out.
Six patients with single enzyme defects underwent liver transplant, including four patients diagnosed with AKR1D1 deficiency, one with 3β-HSD deficiency, and one with CYP7A1 deficiency.
Adverse ReactionsClinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical safety experience with Cholbam consists of:
Trial 1: a non-randomized, open-label, single-arm trial of 50 patients with bile acid synthesis disorders due to SEDs and 29 patients with PDs including Zellweger spectrum disorders. Safety data are available over the 18 years of the trial.Trial 2: an extension trial of 12 new patients (10 SED and 2 PD) along with 31 (21 SED and 10 PD) patients who rolled-over from Trial 1. Safety data are available for 3 years and 11 months of treatment.Adverse events were not collected systematically in either of these trials. Most patients received an oral dose of 10 to 15 mg/kg/day of Cholbam.
Deaths
In Trial 1, among the 50 patients with SEDs, 5 patients aged 1 year or less died, which included three patients originally diagnosed with AKR1D1 deficiency, one with 3β-HSD deficiency and one with CYP7A1 deficiency. The cause of death was attributed to progression of underlying liver disease in every patient.
Of the 29 patients in Trial 1 with PDs including Zellweger spectrum disorders, 12 patients between the ages of 7 months and 2.5 years died. In the majority of these patients (8/12), the cause of death was attributed to progression of underlying liver disease or to a worsening of their primary illness.
Two additional patients in Trial 1 (1 SED and 1 PD) died who had been off study medication for more than one year with the cause of death most likely being a progression of their underlying liver disease. Of the patients who died with disease progression, laboratory testing showed abnormal serum transaminases, bilirubin, or cholestasis on liver biopsy suggesting worsening of their underlying cholestasis.
In Trial 2, among the 31 patients with SED, two patients (1 new patient and 1 who rolled over from Trial 1) died. The cause of death in both cases was unrelated to their primary treatment or progression of their underlying liver disease.
Of the 12 patients with PD in Trial 2, four patients died between the ages of 4 and 8 years (1 new patient and 3 who rolled over from Trial 1). The cause of death in three of these patients was attributed to progression of underlying liver disease or to a worsening of their primary illness.
Worsening Liver Impairment
Seven patients in Trial 1(4 SED and 3 PD) and 3 patients in Trial 2 (1 SED and 2 PD) experienced worsening serum transaminases, elevated bilirubin values, or worsening cholestasis on liver biopsy during treatment.
Common Adverse Reactions
There were 12 adverse reactions reported across 9 patients in the trials, with diarrhea being the most common reaction in approximately 2% of the patient population. All other adverse reactions represented 1% of the patient population. The breakdown by trial follows:
| Adverse Reactions | Trial 1 | Trial 2 * | Overall (%) |
|---|---|---|---|
| *Adverse reactions that occurred in new patients | |||
| Diarrhea | 1 | 2 * | 3 (2%) |
| Reflux Esophagitis | 1 | 0 | 1 (1%) |
| Malaise | 1 | 0 | 1 (1%) |
| Jaundice | 1 | 0 | 1 (1%) |
| Skin lesion | 1 | 0 | 1 (1%) |
| Nausea | 0 | 1 * | 1 (1%) |
| Abdominal Pain | 0 | 1 * | 1 (1%) |
| Intestinal Polyp | 0 | 1 * | 1 (1%) |
| Urinary Tract Infection | 0 | 1 * | 1 (1%) |
| Peripheral Neuropathy | 0 | 1 | 1 (1%) |
Only one of the reactions (peripheral neuropathy) resulted in discontinuation of medication for a patient in Trial 2. An additional five SED patients (3 from Trial 1 and 2 from Trial 2) and 1 PD patient (Trial 1) discontinued medication and withdrew from the study due to a worsening of their primary disease.
The development of symptomatic cholelithiasis requiring cholecystectomy has been reported in a single patient with 3β-HSD deficiency.
Drug Interactions. Effects of other drugs on CholbamDrug interactions with Cholbam mainly relate to agents capable of interrupting the enterohepatic circulation of bile acids.
Inhibitors of Bile Acid Transporters
Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitoring of serum transaminases and bilirubin is recommended.
Bile Acid Binding Resins
Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the efficacy of Cholbam. Take Cholbam at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin [see Dosage and Administration (2.3)] .
Aluminum-Based Antacids
Aluminum-based antacids have been shown to adsorb bile acids in vitro and can reduce the bioavailability of Cholbam. Take Cholbam at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after an aluminum-based antacid [see Dosage and Administration (2.3)] .
USE IN SPECIFIC POPULATIONSPregnancyPregnancy Exposure Registry
There is a pregnancy surveillance program that monitors pregnancy outcomes in women exposed to Cholbam during pregnancy [COCOA Registry (Cholbam: Child and mOther's heAlth)]. Women who become pregnant during Cholbam treatment are encouraged to enroll. Patients or their health care provider should call 1-844-20C-OCOA or 1-844-202-6262 to enroll.
Risk Summary
No studies in pregnant women or animal reproduction studies have been conducted with Cholbam.
Limited published case reports discuss pregnancies in women taking cholic acid for 3β-HSD deficiency resulting in healthy infants. These reports may not adequately inform the presence or absence of drug-associated risk with the use of Cholbam during pregnancy. The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
LactationRisk Summary
Endogenous cholic acid is present in human milk. Clinical lactation studies have not been conducted to assess the presence of Cholbam in human milk, the effects of Cholbam on the breastfed infant, or the effects of Cholbam on milk production. There are no animal lactation data and no data from case reports available in the published literature. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Cholbam and any potential adverse effects on the breastfed infant from Cholbam or from the underlying maternal condition.
Pediatric UseThe safety and effectiveness of Cholbam has been established in pediatric patients 3 weeks of age and older for the treatment of bile acid synthesis disorders due to SEDs, and for adjunctive treatment of patients with PDs including Zellweger spectrum disorders who exhibit manifestations of liver disease, steatorrhea or complications from decreased fat soluble vitamin absorption [see Clinical Studies (14)].
Clinical studies of Cholbam did not include any patients aged 65 years and over. It is not known if elderly patients respond differently from younger patients.
Hepatic ImpairmentDiscontinue treatment with Cholbam if liver function does not improve within 3 months of the start of treatment.
Discontinue treatment with Cholbam at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis [ see Warnings and Precautions (5.1), Overdosage (10) and Nonclinical Toxicology (13.2)]. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
OverdosageConcurrent elevations of serum gamma glutamyltransferase (GGT) and serum alanine aminotransferase (ALT) may indicate Cholbam overdose. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline [see Dosage and Administration (2.2)].
In the event of overdose the patient should be monitored and treated symptomatically.
Cholbam DescriptionCholic acid is a bile acid produced by the liver where it is synthesized from cholesterol. The chemical formula is C 24H 40O 5, the molecular weight is 408.57 and the chemical structure is:
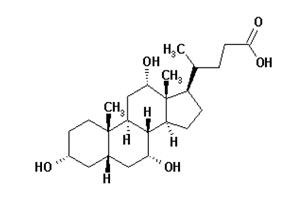
Cholic acid is a white to off-white powder. It is practically insoluble in water and in 0.1 M HCl at 20°C and is sparingly soluble in 0.1 M NaOH at 20°C. It is soluble in glacial acetic acid, alcohols and acetone. A saturated solution in water at 20°C has a pH of 4.4.
Cholbam capsules contain 50 mg or 250 mg of cholic acid as the active ingredient in size 2 Swedish orange or size 0 white opaque gelatin capsules, respectively. Inactive ingredients in Cholbam include silicified microcrystalline cellulose, magnesium stearate and hard gelatin capsules. The size 2 shells contain gelatin, red iron oxide and titanium dioxide and the size 0 shells contain gelatin and titanium dioxide. Cholbam is administered orally.
Cholbam - Clinical PharmacologyMechanism of ActionCholic acid is a primary bile acid synthesized from cholesterol in the liver. In bile acid synthesis disorders due to SEDs in the biosynthetic pathway, and in PDs including Zellweger spectrum disorders, deficiency of primary bile acids leads to unregulated accumulation of intermediate bile acids and cholestasis. Bile acids facilitate fat digestion and absorption by forming mixed micelles, and facilitate absorption of fat-soluble vitamins in the intestine.
Endogenous bile acids including cholic acid enhance bile flow and provide the physiologic feedback inhibition of bile acid synthesis. The mechanism of action of cholic acid has not been fully established; however, it is known that cholic acid and its conjugates are endogenous ligands of the nuclear receptor, farnesoid X receptor (FXR). FXR regulates enzymes and transporters that are involved in bile acid synthesis and in the enterohepatic circulation to maintain bile acid homeostasis under normal physiologic conditions.
PharmacokineticsOrally administered cholic acid is subject to the same metabolic pathway as endogenous cholic acid.
Cholic acid is absorbed by passive diffusion along the length of the gastrointestinal tract. Once absorbed, cholic acid enters into the body's bile acid pool and undergoes enterohepatic circulation mainly in conjugated forms.
In the liver, cholic acid is conjugated with glycine or taurine by bile acid-CoA synthetase and bile acid-CoA: amino acid N-acetyltransferase. Conjugated cholic acid is actively secreted into bile mainly by the Bile Salt Efflux Pump (BSEP), and then released into the small intestine, along with other components of bile.
Conjugated cholic acid is mostly re-absorbed in the ileum mainly by the apical-sodium-dependent-bile acid transporter, passed back to the liver by transporters including sodium-taurocholate cotransporting polypeptide and organic anion transport protein and enters another cycle of enterohepatic circulation. Any conjugated cholic acid not absorbed in the ileum passes into the colon where deconjugation and 7-dehydroxylation are mediated by bacteria to form cholic acid and deoxycholic acid which may be re-absorbed in the colon or excreted in the feces. The loss of cholic acid is compensated by de-novo synthesis of cholic acids from cholesterol to maintain the bile acid pool in healthy subjects.
Nonclinical ToxicologyCarcinogenesis, Mutagenesis, Impairment of FertilityCarcinogenicity, genetic toxicology, and nonclinical fertility studies have not been performed with cholic acid.
Animal Toxicology and/or PharmacologyIn the PEX2 -/- mouse model of peroxisomal disorders, feeding with a combination of cholic acid and ursodeoxycholic acid normalized C 24 bile acid concentrations in bile to that of untreated control animals. Although growth was only mildly improved, there was near complete normalization of stool fat content, resolution of steatorrhea, and improved survival. Bile acid feeding reduced the number of cholestatic deposits in bile ducts and alleviated cholangitis, but exacerbated the degree of hepatic steatosis and mitochondrial and cellular damage in the peroxisome-deficient livers of these animals.
Clinical StudiesBile Acid Synthesis Disorders due to Single Enzyme DefectsThe effectiveness of Cholbam at dosages of 10 to 15 mg/kg per day in patients with SEDs was assessed in:
Trial 1: a non-randomized, open-label, single-arm trial in 50 patients over an 18 year period.Trial 2: an extension trial of 12 new patients along with 21 patients who rolled-over from Trial 1 (n=33 total). Efficacy data are available for 21 months of treatment.A published case series of 15 patients.Enrollment criteria in Trials 1 and 2 were based on abnormal urinary bile acid by Fast Atom Bombardment ionization - Mass Spectrometry (FAB-MS) analysis.
Pre- and post-treatment liver biopsies were performed in a limited number of patients. Documentation of adherence to treatment, concomitant medications and response to treatment were incomplete during Trial 1. Additional interventions in some patients included supplementation with fat-soluble vitamins, as dictated by the patient's clinical signs and symptoms.
Trials 1 and 2
On average, patients were 4 years of age at the start of cholic acid treatment (range three weeks to 36 years). The majority of patients were treated for an average of 310 weeks (6 years). Patient ages at the end of treatment ranged from 19 to 36 years.
These trials were carried out over many years and data are not available on all patients. Thirty-nine patients in Trial 1 and 5 new patients in Trial 2 received at least one dose of Cholbam and had sufficient data available to assess baseline liver function and effects of Cholbam treatment. A responder analysis was performed to determine the response to treatment with Cholbam.
Response to Cholbam treatment was assessed by the following laboratory criteria:
ALT or AST values reduced to less than 50 U/L, or baseline levels reduced by 80%;total bilirubin values reduced to less than or equal to 1 mg/dL; andno evidence of cholestasis on liver biopsy;and the following clinical criteria:
body weight increased by 10% or stable at greater than the 50th percentile; andsurvival for greater than 3 years on treatment or alive at the end of Trial 2Cholbam responders were defined as patients who either:
met at least two laboratory criteria and were alive at the last follow-up; ormet at least one laboratory criterion, had increased body weight and were alive at the last follow-up.Overall, 28 of 44 patients (64%) were responders. The breakdown by defect type is as follows:
| Single Enzyme Defect | Responders/Number Treated (%) |
|---|---|
| *N/A indicates no evaluable patients in the defect subgroup represented. | |
| 3β-HSD | 22/37 (59%) |
| AKR1D1 | 3/4 (75%) |
| CTX | 2/2 (100%) |
| AMACR | 1/1 (100%) |
| CYP7A1 | N/A * |
| Smith-Lemli-Opitz | N/A * |
Among SED responsive patients, 45% of the responders met the two clinical criteria plus 1 to 3 laboratory criteria and 55% met the weight criteria.
Only six patients had pre- and post-treatment liver biopsies in Trial 1. Where biopsies were available, pre-treatment biopsies showed varying degrees of inflammation, bridging fibrosis, and giant cell formation. Post-treatment biopsies generally showed reduced or absent inflammation and reduced or absent giant cell formation. Fibrosis remained but did not progress.
It is difficult to evaluate long term survival in patients with SEDs since there is little natural history survival data for comparison. Overall, 41 of 62, or 67%, of patients with SEDs survived greater than 3 years from trial entry. Thirteen of these 41 patients, or 32%, were "long-term" survivors (range of 10 to 24 years on treatment).
Four patients in Trial 1 underwent liver transplant, including two patients diagnosed with AKR1D1 deficiency, one with 3β-HSD deficiency, and one with CYP7A1 deficiency and two patients in Trial 2, both with AKR1D1.
Cholbam's effects on extrahepatic manifestations of SEDs, such as neurologic symptoms are not established.
Case Series
A published report of a case series described 15 patients with SEDs; thirteen were diagnosed with 3β-HSD deficiency and two with AKR1D1 deficiency by mass spectrometry and gene sequencing. All patients were treated with cholic acid with a median duration of treatment of 12.4 years (range 5.6 to 15 years). Therapy started at a median age of 3.9 years (range 0.3 to 13.1 years). The mean dose at the start of cholic acid treatment was 13 mg/kg and the mean dose at last follow up was 6 mg/kg. Eight patients were initially treated with oral ursodeoxycholic acid prior to receiving a diagnosis of bile acid synthesis defect, after which they were switched to cholic acid. Initial signs and symptoms included jaundice, hepatosplenomegaly, steatorrhea, or symptoms related to deficiency of a fat soluble vitamin (K, D or E).
Of the 8 patients who received ursodeoxycholic acid initially, the six with 3β-HSD deficiency demonstrated mild clinical improvement. Following treatment with cholic acid, all patients experienced resolution of their pre-existing jaundice and steatorrhea, and all but one experienced resolution of hepatosplenomegaly. Weight and height improved and sexual maturation progressed normally in all patients. Liver biopsies were performed in 14 patients after at least 5 years of cholic acid treatment and all showed resolution of cholestasis. In one patient with 3β-HSD deficiency, biliary bile acid analysis while on cholic acid therapy showed enrichment of the bile with cholic acid.
Peroxisomal Disorders including Zellweger Spectrum DisordersThe effectiveness of Cholbam at a dosage of 10 to 15 mg/kg per day in patients with PDs including Zellweger spectrum disorders was assessed in patients in the same trials described in section 14.1.
Trial 1 treated 29 patients with PDs over an 18 year period.Trial 2 treated 2 new patients along with 10 patients who rolled-over from Trial 1 (n=12 total). Efficacy data are available from Trial 2 for 21 months of treatment.Additional efficacy data were obtained from published case reports of 3 patients.Enrollment criteria in Trials 1 and 2 were based on abnormal urinary bile acids analysis by Fast Atom Bombardment ionization - Mass Spectrometry (FAB-MS) and a neurologic exam. Most patients received concomitant DHA (docosahexaenoic acid) and Vitamins A, D, E and K. Documentation of adherence to treatment, concomitant medications and response to treatment were incomplete during Trial 1.
Trials 1 and 2
The majority of patients (80%, 25/31) were less than 2 years of age at the start of Cholbam treatment (range 3 weeks to 10 years). The majority of patients were treated for an average of 254 weeks (4.8 years).
Sufficient data were available to assess baseline liver function and effects of Cholbam treatment in 23 patients in Trial 1 and in one new patient in Trial 2. A responder analysis was performed in the patients who had received at least one dose of Cholbam and had sufficient data available to assess baseline liver impairment.
Response to Cholbam treatment was assessed by the following laboratory criteria:
ALT or AST values reduced to less than 50 U/L, or baseline levels reduced by 80%;total bilirubin values reduced to less than or equal to 1 mg/dL; andno evidence of cholestasis on liver biopsy;and the following clinical criteria:
body weight increased by 10% or stable at greater than the 50th percentile; andsurvival for greater than 3 years on treatment or alive at the end of Trial 2Cholbam responders were defined as patients who either:
met at least two laboratory criteria and were alive at the last follow-up; ormet at least one laboratory criterion, had increased body weight and were alive at the last follow-up.Overall, 11 of 24 patients (46%) were responders. The breakdown by disorder is as follows:
| Peroxisomal Disorder | Responders/Number Treated (%) |
|---|---|
| Neonatal Adrenoleukodystropyhy | 3/6 (50%) |
| Generalized Peroxisomal Disorder | 1/1 (100%) |
| Refsum Disease | 3/4 (75%) |
| Zellweger Syndrome | 3/8 (38%) |
| Peroxisomal Disorder, Type Unknown | 1/5 (20%) |
Among responsive patients with PDs, 38% of the responders met the two clinical criteria plus 1 to 3 laboratory criteria and 63% met the weight criteria. There were no PD patients that underwent liver transplant.
No evidence of improvement in survival over that seen in historical controls could be demonstrated from the data presented. Overall, 13 of 31, or 42%, of patients survived greater than 3 years from the time of trial entry. Eight of these 13 patients, or 62% were "long-term" survivors (range of 10 to 17 years on treatment).
Nine patients had both pre- and post-treatment liver biopsies. One patient showed improvement in histology, while the majority of patients remained unchanged. Two patients demonstrated worsening histology, which was consistent with a worsening of other liver laboratory parameters (bilirubin, serum transaminase values).
Cholbam's effects on extrahepatic manifestations of PDs including Zellweger spectrum disorders, such as neurologic symptoms are not established.
One patient, who did not have cholestasis on pre-treatment liver biopsy, developed cholestasis on treatment with Cholbam and subsequently died.
Case Reports
In case reports from the literature, a 6 month old patient with Zellweger syndrome treated with a combination of cholic and chenodeoxycholic acids experienced normalization of serum transaminases and bilirubin, improvement in liver histology, reduced serum and urinary atypical bile acid intermediates, and improvement in steatorrhea and growth. Two patients with Zellweger syndrome treated with oral bile acids showed decreased serum transaminases.
How Supplied/Storage and Handling50 mg Capsules
Cholbam capsules are available as two-piece gelatin capsules with a Swedish orange cap imprinted with "50mg" and Swedish orange body with imprinted with "ASK001". The capsules contain a white or off-white powder and are supplied in bottles of:
90 capsules (NDC 45043-001-02)250 mg Capsules
Cholbam capsules are available as two-piece gelatin capsules with a white cap imprinted with "250mg" and white body with imprinted with "ASK002". The capsules contain a white or off-white powder and are supplied in bottles of:
90 capsules (NDC 45043-002-02)Storage and Handling
Store at 20–25°C (69-77°F), excursions permitted between 15-30°C (59-86°F). [see USP Controlled Room Temperature].
Patient Counseling InformationExacerbation of Liver Impairment
Advise patients that they will need to undergo laboratory testing periodically while on treatment to assess liver function.Advise patients that Cholbam may worsen liver impairment and that they should immediately report to their health care provider any symptoms associated with liver impairment (e.g., skin or the whites of eyes turn yellow, urine turns dark or brown [tea colored], pain on the right side of stomach, bleeding or bruising occurs more easily than normal, or increased lethargy)Administration
Advise patients:
to take Cholbam with food.to take Cholbam at least one hour before or 4 to 6 hours after taking a bile acid binding resin or an aluminum-based antacid.not to crush or chew the capsules.for infants and children who cannot swallow capsules, the capsules can be opened and the contents mixed with either infant formula or expressed breast milk (for younger children), or soft food such as mashed potatoes or apple puree (for older children and adults) in order to mask any unpleasant taste:Hold the capsule over the prepared liquid/food, gently twist open, and allow the contents to fall into the liquid/food.Mix the entire capsule contents with one or two tablespoonfuls (15 mL to 30 mL) of infant formula, expressed breast milk, or soft food such as mashed potatoes or apple puree.Stir for 30 seconds.The capsule contents will remain as fine granules in the milk or food, and will not dissolve.Administer the mixture immediately.Pregnancy Registry:
Advise patients there is a pregnancy surveillance program that monitors pregnancy outcomes in women exposed to Cholbam during pregnancy [see Use in Specific Populations (8.1)] .
Cholbam™ is a trademark of Retrophin, Inc.
Revised: March 2015
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
Manufactured for:
Manchester Pharmaceuticals, Inc. A wholly owned subsidiary of Retrophin, Inc. San Diego, CA 92130
For further information, please call 844-246-5226
NDC 45043- 001-02
90 capsules
Cholbam™
(cholic acid) capsules
50 mg
Rx Only
Manufactured for:
Manchester Pharmaceuticals, Inc.,
San Diego, CA 92130
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
For product information please call 844-246-5226.
NDC 45043- 002-02
90 capsules
Cholbam™
(cholic acid) capsules
250 mg
Rx Only
Manufactured for:
Manchester Pharmaceuticals, Inc.,
San Diego, CA 92130
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
For product information please call 844-246-5226.
|
Cholbam cholic acid capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
Cholbam cholic acid capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Retrophin, Inc (832417641) |
| Registrant - Retrophin, Inc (965454502) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Patheon France S.A.S | 543127229 | api manufacture(45043-001, 45043-002) | |
Medical Disclaimer