

Triptodur 醋酸曲普瑞林缓释针剂




| 通用中文 | 醋酸曲普瑞林缓释针剂 | 通用外文 | Triptorelin Acetate For Injection |
| 品牌中文 | 品牌外文 | Triptodur | |
| 其他名称 | Decapeptyl Depot GONAPEPTYL DEPOT Trelstar | ||
| 公司 | Arbor(Arbor) | 产地 | 美国(USA) |
| 含量 | 22.5mg | 包装 | 1瓶/盒 |
| 剂型给药 | 肌肉注射 粉针剂 | 储存 | 室温 |
| 适用范围 | Triptodur适用于治疗2岁以上的中枢性性早熟(CPP)的小儿患者。 | ||
| 通用中文 | 醋酸曲普瑞林缓释针剂 |
| 通用外文 | Triptorelin Acetate For Injection |
| 品牌中文 | |
| 品牌外文 | Triptodur |
| 其他名称 | Decapeptyl Depot GONAPEPTYL DEPOT Trelstar |
| 公司 | Arbor(Arbor) |
| 产地 | 美国(USA) |
| 含量 | 22.5mg |
| 包装 | 1瓶/盒 |
| 剂型给药 | 肌肉注射 粉针剂 |
| 储存 | 室温 |
| 适用范围 | Triptodur适用于治疗2岁以上的中枢性性早熟(CPP)的小儿患者。 |
Generic Name: triptorelin
Dosage Form: extended-release injection
Medically reviewed by Drugs.com. Last updated on Apr 1, 2019.
OverviewSide EffectsDosageProfessionalInteractionsMoreTriptodur is indicated for the treatment of pediatric patients 2 years of age and older with central precocious puberty (CPP).
Triptodur Dosage and AdministrationDosing InformationMust only be administered by a healthcare provider.
The dosage of Triptodur is 22.5 mg reconstituted with accompanying diluent (sterile water) 2 mL, and administered as a single intramuscular injection once every 24 weeks.
Triptodur treatment should be discontinued at the appropriate age of onset of puberty at the discretion of the physician.
MonitoringMonitor response to Triptodur with LH levels after a GnRH or GnRH agonist stimulation test, basal LH, or serum concentration of sex steroid levels beginning 1 to 2 months following initiation of therapy, during therapy as necessary to confirm maintenance of efficacy, and with each subsequent dose.
Measure height (for calculation of growth rate) every 3-6 months and monitor bone age periodically.
Noncompliance with drug regimen or inadequate dosing may result in inadequate control of the pubertal process with gonadotropins and/or sex steroids increasing above prepubertal levels. If the dose of Triptodur is not adequate switching to an alternative GnRH agonist for the treatment of CPP with the ability for dose adjustment may be necessary.
Reconstitution and Administration Instructions*** Triptodur should be injected immediately after reconstitution in accordance with the detailed instructions below. ***
Please read these instructions completely before you begin.
Use appropriate aseptic technique for preparation and administration.Screw the plunger rod into the barrel end of the prefilled sterile water diluent syringe.
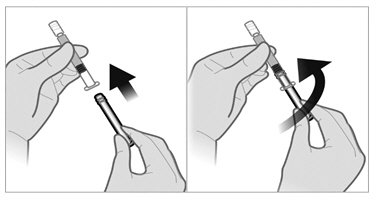
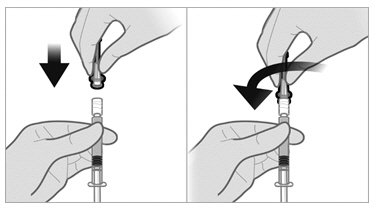
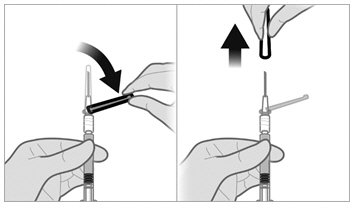
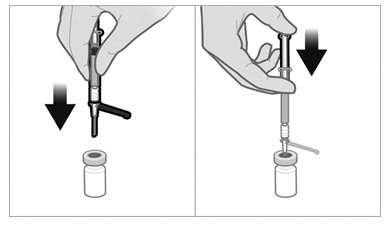
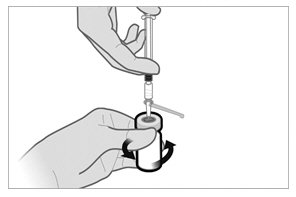
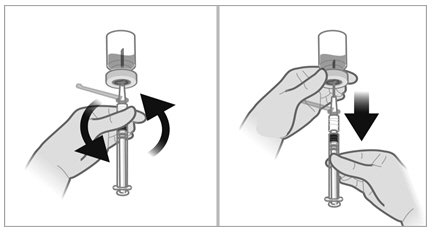
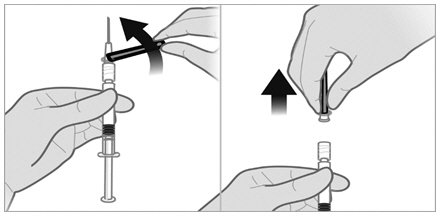
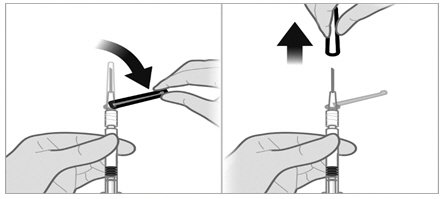
To minimize the risk of needle blockage during the injection, ensure that the preparation of the injection is not interrupted and/or the mixed suspension syringe is not put aside because the suspension will sediment quickly.
a.If the suspension appears milky and homogenous without visible aggregates or precipitates, then prime the needle and administer the suspension immediately.Do not prime the needle if the suspension does not appear milky and homogenousIf the suspension does not appear milky and homogenous, continue with an up and down agitationPrime the needle immediately prior to administration of the homogenous suspension
For extended-release injectable suspension: 22.5 mg of triptorelin as a lyophilized white to slightly yellow powder cake in a single-dose vial for reconstitution with the co-packaged 2 mL of diluent (sterile water) for injection.
ContraindicationsHypersensitivity: Triptodur is contraindicated in individuals with a known hypersensitivity to triptorelin, any other component of the product, or other GnRH agonists or GnRH [see Adverse Reactions (6.2)].Pregnancy: Triptodur may cause fetal harm [see Use in Specific Populations (8.1)].Warnings and PrecautionsInitial Rise of Gonadotropins and Sex Steroid LevelsDuring the early phase of initial therapy or after subsequent doses, gonadotropins and sex steroids may rise above baseline because of a transient stimulatory effect of the drug [see Clinical Pharmacology (12.2)]. Therefore, a transient increase in clinical signs and symptoms of puberty, including vaginal bleeding, may be observed during the first weeks of therapy or after subsequent doses.
Psychiatric EventsPsychiatric events have been reported in patients taking GnRH agonists, including triptorelin. Postmarketing reports with this class of drugs include symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms during treatment with Triptodur [see Adverse Reactions (6)].
ConvulsionsPostmarketing reports of convulsions have been observed in patients receiving GnRH agonists, including triptorelin. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above [see Adverse Reactions (6)].
Adverse ReactionsThe following serious adverse reactions are described here and elsewhere in the label:
Initial Rise of Gonadotropins and Sex Steroid Levels [see Warnings and Precautions (5.1)]Psychiatric Events [see Warnings and Precautions (5.2)]Convulsions [see Warnings and Precautions (5.3)]Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Triptodur was evaluated in one uncontrolled, open-label single-arm clinical trial in which 44 children with central precocious puberty received two doses of Triptodur and were observed for 12 months. The median age of the study population was 8 years (range 2-9 years) at treatment start; 88.6% of subjects were female, 59.1% were White, 27.3% were Black and 4.5% were Asian. Table 1 shows all the adverse reactions that occurred in at least 2 patients (≥4.5%) during the open-label single-arm trial.
| Adverse Reactions |
Number of Patients Reporting Event (%) (Total N=44) |
|---|---|
| *Injection site reactions are presented separately†Includes % of patients with vaginal bleeding or menstrual disorder ("menstrual cycle returned") in 39 females out of N=44. | |
| Infections & Infestations | |
| Bronchitis | 2 (4.5) |
| Gastroenteritis | 3 (6.8) |
| Influenza | 2 (4.5) |
| Nasopharyngitis | 6 (13.6) |
| Otitis externa | 2 (4.5) |
| Pharyngitis | 2 (4.5) |
| Sinusitis | 2 (4.5) |
| Upper respiratory tract infection | 4 (9.1) |
| Nervous System Disorders | |
| Headache | 6 (13.6) |
| Reproductive System & Breast Disorders | |
| Menstrual (Vaginal bleeding)† | 3 (7.7) |
| Respiratory, Thoracic & Mediastinal Disorder | |
| Cough | 3 (6.8) |
| Vascular Disorders | |
| Hot flush | 2 (4.5) |
Other Selected Adverse Reactions:
Injection Site Reactions
Injection site reactions occurring in patients immediately and/or 2 hours after injection include pain (45%), redness (14%), pruritus (2.3%) and swelling (2.3%).
Psychiatric Disorders
Anxiety (2.3%) and mood altered (2.3%)
Postmarketing ExperienceBecause these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions were reported from postmarketing experience of triptorelin in patients with CPP:
Hypersensitivity Reactions: Anaphylactic shock, anaphylactoid reaction, angioedema, urticaria.
Cardiovascular: Hypertension.
Psychiatric: Emotional lability, such as crying, irritability, impatience, anger, and aggression, has been observed with GnRH agonists, including triptorelin [see Warnings and Precautions (5.2)]; Depression, including rare reports of suicidal ideation and attempt, has been reported for GnRH agonists in children treated for CPP. Many, but not all, of these patients had a history of psychiatric illness or other comorbidities with an increased risk of depression.
Nervous System: Convulsions [see Warnings and Precautions (5.3)]
Vision Disorders: Visual impairment, visual disturbance
Drug InteractionsDrug-Drug InteractionsResults of in vitro studies show that drug-drug interactions with triptorelin are unlikely [see Clinical Pharmacology (12.3)]. However, in the absence of relevant data and as a precaution, hyperprolactinemic drugs should not be used concomitantly with triptorelin since hyperprolactinemia reduces the number of pituitary GnRH receptors.
Drug-Laboratory Test InteractionsAdministration of Triptodur results in suppression of the pituitary-gonadal system.
The effect of Triptodur on pituitary and gonadal function is expected to disappear within six to twelve months after treatment discontinuation. Therefore, diagnostic tests of pituitary gonadotropic and gonadal functions conducted during treatment or after discontinuation of treatment may be affected.
USE IN SPECIFIC POPULATIONSPregnancyRisk Summary
Triptodur is contraindicated in women who are pregnant [see Contraindications (4)] since expected hormonal changes that occur with Triptodur treatment increase the risk for pregnancy loss. Available data with triptorelin use in pregnant women are insufficient to determine a drug-associated risk of adverse developmental outcomes. Based on mechanism of action in humans and findings of increased pregnancy loss in animal studies, Triptodur may cause fetal harm when administered to pregnant women. Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% -20%, respectively.
Data
Animal Data
In pregnant rats administered triptorelin at doses of 2, 10, and 100 mcg/kg/day during the period of organogenesis, maternal toxicity (decrease in body weight) and embryo-fetal toxicities (pre-implantation loss, increased resorption, and reduced number of viable fetuses) were observed at 100 mcg/kg, approximately 4 times the clinical dose based on body surface area. No embryonic and fetal developmental toxicities were observed in mice at doses up to 4 times the clinical dose. Teratogenic effects were not observed in viable fetuses in rats or mice.
Risk Summary
There are no data on the presence of triptorelin in human milk, or the effects of the drug on the breastfed infant, or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Triptodur and any potential adverse effects on the breastfed child from Triptodur or from the underlying maternal condition.
Pediatric UseThe safety and effectiveness of Triptodur have been established in pediatric patients 2 years of age and older based on a single-arm open-label study of 44 children 2-9 years of age with CPP [see Clinical Studies (14)]. The safety and effectiveness of Triptodur have not been established in pediatric patients less than 2 years old.
Renal ImpairmentTriptodur has not been studied in children with renal impairment. Adult subjects with renal impairment had higher exposure than young healthy adult males [see Clinical Pharmacology (12.3)].
Hepatic ImpairmentTriptodur has not been studied in children with hepatic impairment. Adult subjects with hepatic impairment had higher exposure than young healthy adult males [see Clinical Pharmacology (12.3)].
OverdosageThere is no experience with overdosage in clinical trials of triptorelin. If overdosage occurs, therapy should be discontinued and appropriate supportive and symptomatic treatment administered.
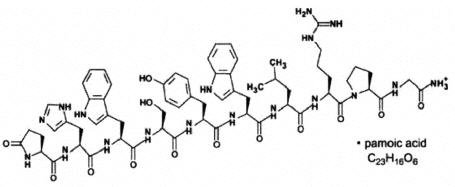
Triptodur DescriptionTriptodur contains the pamoate salt of triptorelin, a synthetic decapeptide analog of naturally occurring gonadotropin-releasing hormone (GnRH or LHRH). The chemical name of triptorelin pamoate is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-tryptophyl-L-leucyl-L-arginyl-L-prolylglycine amide (pamoate salt). The molecular weight is 1699.9 and the structural formula is:

Triptodur for extended release injectable suspension for intramuscular use is provided as a sterile, lyophilized, biodegradable microgranule formulation in a single-dose vial, co-packaged with a syringe containing 2 mL diluent (sterile water) for injection for reconstitution of the lyophilisate. The triptorelin formulation is comprised of 22.5 mg triptorelin (equivalent to 31 mg triptorelin pamoate), poly-d,l-lactide-co-glycolide (183 mg), mannitol (74 mg), carboxymethylcellulose sodium (26 mg), and polysorbate 80 (1.7 mg). When 2 mL diluent (sterile water) for injection is added to the vial containing Triptodur and mixed, a suspension is formed which is intended as a single intramuscular injection.
Triptodur - Clinical PharmacologyMechanism of ActionTriptorelin is a GnRH agonist.
PharmacodynamicsFollowing the first administration, there is a transient surge in circulating levels of LH, FSH, testosterone, and estradiol [see Warnings and Precautions (5.2)]. After chronic and continuous administration, by 4 weeks after initiation of therapy, a sustained decrease in LH and FSH secretion and marked reduction in sex steroids are observed.
PharmacokineticsAbsorption
After an initial intramuscular Triptodur 22.5 mg injection and a second 22.5 mg intramuscular injection 24 weeks later in children 2 to 9 years old with CPP, triptorelin peaked 4 hours postdose with a geometric mean Cmax of 39.9 and 36.5 ng/mL, respectively. No apparent accumulation of triptorelin occurred after the second injection. Absorption occurred in two phases, a burst phase followed by a maintenance release phase. In children with CPP, following the burst phase after the first 22.5 mg injection, geometric mean serum triptorelin levels were 0.11, 0.17, 0.05 and 0.03 ng/mL at Months 1, 2, 3, and 6, respectively.
Distribution
There is no evidence that triptorelin, at clinically relevant concentrations, binds to plasma proteins.
Elimination
Metabolism
The metabolism of triptorelin in humans is unknown, but is unlikely to involve hepatic microsomal enzymes (cytochrome P450). Thus far no metabolites of triptorelin have been identified. Pharmacokinetic data suggest that C-terminal fragments produced by tissue degradation are either completely degraded in the tissues, or rapidly degraded in plasma, or cleared by the kidneys.
Excretion
Triptorelin is eliminated by both the liver and the kidneys. Following intravenous administration of 0.5 mg triptorelin peptide to six healthy male volunteers with a creatinine clearance of 149.9 mL/min, 41.7% of the dose was excreted in urine as intact peptide with a total triptorelin clearance of 211.9 mL/min. This percentage increased to 62.3% in patients with liver disease who have a lower creatinine clearance (89.9 mL/min). It has also been observed that the nonrenal clearance of triptorelin (patient anuric, Clcreat = 0) was 76.2 mL/min, thus indicating that the nonrenal elimination of triptorelin is mainly dependent on the liver.
Specific Populations
Renal Impairment
After intravenous bolus injection of 0.5 mg triptorelin in adults, the two distribution half-lives were unaffected by renal impairment. However, renal insufficiency led to a decrease in total triptorelin clearance proportional to the decrease in creatinine clearance as well as increases in volume of distribution and consequently, an increase in the elimination half-life. Adult male subjects with moderate or severe renal impairment had approximately 2-fold higher exposure (AUC values) than young healthy adult males (see Table 1) [see Use in Specific Populations (8.6)].
Hepatic Impairment
After intravenous bolus injection of 0.5 mg triptorelin in adults, the two distribution half-lives were unaffected by hepatic impairment. In adult males with hepatic insufficiency, triptorelin clearance was reduced and exposure (AUC) was increased 3.7-fold compared to young healthy adult males (Table 2) [see Use in Specific Populations (8.7)].
| Group |
Cmax (ng/mL) |
AUCinf (h∙ng/mL) |
Clp (mL/min) |
Clrenal (mL/min) |
t1/2 (h) |
Clcreat (mL/min) |
|---|---|---|---|---|---|---|
| 6 healthy male volunteers | 48.2 ±11.8 | 36.1 ±5.8 | 211.9 ±31.6 | 90.6 ±35.3 | 2.81 ±1.21 | 149.9 ±7.3 |
| 6 males with moderate renal impairment | 45.6 ±20.5 | 69.9 ±24.6 | 120.0 ±45.0 | 23.3 ±17.6 | 6.56 ±1.25 | 39.7 ±22.5 |
| 6 males with severe renal impairment | 46.5 ±14.0 | 88.0 ±18.4 | 88.6 ±19.7 | 4.3 ±2.9 | 7.65 ±1.25 | 8.9 ±6.0 |
| 6 males with liver disease | 54.1 ±5.3 | 131.9 ±18.1 | 57.8 ±8.0 | 35.9 ±5.0 | 7.58 ±1.17 | 89.9 ±15.1 |
Drug-Drug Interactions
In Vitro Assessment of Drug Interactions
Drug Metabolizing Enzyme Inhibition
Triptorelin did not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19 or 2D6, or CYP 3A4/5 at clinically relevant concentrations.
Drug Metabolizing Enzyme Induction
In fresh human hepatocytes from three human donors, triptorelin did not induce CYP1A2 or CYP3A4/5 activity.
Transporters
Triptorelin was a poor P-gp substrate and had no inhibitory effect toward P-gp.
Carcinogenesis was evaluated in an 18-month study in mice and a 24-month study in rats. In rats, triptorelin doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.2, 0.8, and 4 times the estimated human monthly dose based on body surface area) resulted in increased mortality with a drug treatment period of 13 to 19 months. The incidences of benign and malignant pituitary tumors and histosarcomas were increased in a dose-related manner. There were no treatment-related tumors in mice at exposure up to 4-fold higher than the estimated human monthly dose based on body surface area.
Mutagenicity studies performed with triptorelin using bacterial and mammalian systems (in vitro Ames test and chromosomal aberration test in CHO cells and an in vivo mouse micronucleus test) provided no evidence of mutagenic potential.
After 60 days of subcutaneous treatment followed by a minimum of four estrus cycles prior to mating, triptorelin at doses of 2, 20, and 200 mcg/kg (approximately 0.07, 0.7, and 7 times the estimated human daily dose based on body surface area) or two monthly injections as slow release microspheres (~20 mcg/kg/day) had no effect on the fertility or general reproductive function of female rats.
No studies were conducted to assess the effect of triptorelin on male fertility.
Clinical StudiesIn a single-arm open-label study, 44 children 2 to 9 years of age with CPP, 39 females and 5 males, all naïve to previous GnRH agonist treatment, were administered Triptodur 22.5 mg at a dosing interval of 24 weeks. Subjects were evaluated over two dosing intervals for a total of 12 months.
Triptodur 22.5 mg suppressed pituitary release of LH and FSH and, consequently, gonadal secretion of estradiol in girls and testosterone in boys (Table 3). At all timepoints evaluated, ≥93% of children achieved LH suppression to prepubertal levels (i.e., serum LH ≤5 IU/L 30 minutes after GnRH agonist stimulation), ≥79% of girls achieved prepubertal levels of estradiol (i.e., <20 pg/mL), and ≥80% of boys achieved prepubertal levels of testosterone (i.e., <30 ng/dL). Triptodur arrested or reversed progression of clinical signs of puberty with 95% of children showing no increase in the bone age/chronological age ratio, and 89% showing stabilization of sexual maturation at Month 12.
| Endpoint | % (n/N) of Children Achieving Endpoint | |||||
|---|---|---|---|---|---|---|
| Month 1 | Month 2 | Month 3 | Month 6 | Month 9 | Month 12 | |
| *Intent-to-Treat population†Primary efficacy endpoint‡Bone Age/Chronological Age | ||||||
| % with prepubertal LH (GnRH-stim LH ≤5 IU/L) |
95% (42/44) |
95% (42/44) |
95% (42/44) |
93%† (41/44) |
95% (42/44) |
98% (43/44) |
| % girls with prepubertal estradiol (<20 pg/mL) |
87% (34/39) |
89% (34/38) |
92% (36/39) |
79% (31/39) |
82% (32/39) |
79% (31/39) |
| % boys with prepubertal testosterone (<30 ng/dL) |
80% (4/5) |
80% (4/5) |
100% (5/5) |
100% (5/5) |
80% (4/5) |
80% (4/5) |
| % with no increase in BA/CA‡ ratio vs. baseline |
64% (28/44) |
95% (42/44) |
||||
| % achieving stabilization of sexual maturation |
91% (40/44) |
89% (39/44) |
||||
| % girls with regression of uterine length |
69% (27/39) |
77% (30/39) |
||||
| % boys with no progression in testis volumes |
100% (5/5) |
100% (5/5) |
||||
Following the second Triptodur injection, 22 children (all girls) were assessed for evidence of an acute-on-chronic phenomenon (i.e., increase in basal LH >5 IU/L or serum estradiol level >20 pg/mL 48 hours after the second triptorelin injection). Of these, one girl who achieved prepubertal hormone levels prior to the second injection showed biochemical evidence of acute-on-chronic phenomenon [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Each Triptodur 22.5 mg single-use kit (NDC 24338-150-20) contains:
One single-dose brown-tinted vial of Triptodur 22.5 mg (NDC 24338-150-01) with a Flip-Off seal containing sterile lyophilized white to slightly yellow powder cakeOne sterile, glass syringe with Luer Lock prefilled with 2 mL of diluent (sterile water) for injection (NDC 24338-150-02)Two sterile 21 gauge, 1½" needles (thin-wall) with safety coverOne Package InsertStore at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. Do not freeze.
Patient Counseling InformationAdvise the patient to read the FDA-approved patient labeling (Patient Information and Medication Guide).
Hypersensitivity Reactions
Inform caregivers that anaphylactic shock, hypersensitivity, and angioedema have been reported with triptorelin use and to immediately seek medical attention if any hypersensitivity reaction occurs.
Symptoms after Initial Triptodur Administration
Inform caregivers that during the first weeks after the first Triptodur injection, signs of puberty may occur such as vaginal bleeding [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)]. Caregivers should notify the physician if these symptoms continue beyond the second month after Triptodur administration.
Psychiatric Events
Inform caregivers that symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression have been observed in patients receiving GnRH agonists, including triptorelin. Alert caregivers to the possibility of development or worsening of psychiatric symptoms, including depression, during treatment with Triptodur [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)].
Convulsions
Inform caregivers that reports of convulsions have been observed in patients receiving GnRH agonists, including triptorelin. Patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions may be at increased risk [see Warnings and Precautions (5.3)].
Pregnancy is Contraindicated
Triptodur is contraindicated in pregnancy. If the patient becomes pregnant while taking the drug, the patient should be informed of the potential risk to fetus [see Use in Specific Populations (8.1)].
Compliance with the Dosing Schedule
Inform caregivers about the importance of adherence to the Triptodur dosing schedule of one injection every 24 weeks. Patients should not miss or delay a scheduled dose.
Manufactured for:
Arbor Pharmaceuticals, LLC
Atlanta, GA 30328
Manufactured by:
Debiopharm Research & Manufacturing SA
CH-1920 Martigny, Switzerland
© 2018 Arbor Pharmaceuticals, LLC
Triptodur is a registered trademark of Debiopharm International SA.
Issue: October 2018
TRIP-PI-05
|
MEDICATION GUIDE Triptodur® [TRIP-toe-der] (triptorelin) for extended-release injectable suspension, for intramuscular use |
|||
|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Issue: 10/2018 | ||
|
What is the most important information I should know about Triptodur?In the first few weeks after your child receives their first Triptodur injection or after additional injections, Triptodur can cause a brief increase in some hormones. During this time you may notice more signs of puberty in your child, including vaginal bleeding. Call your child's doctor if signs of puberty continue after 2 months of receiving Triptodur.Some people taking gonadotropin releasing hormone (GnRH) agonists like Triptodur have had new or worsened mental (psychiatric) problems. Mental (psychiatric) problems may include emotional symptoms such as:cryingirritabilityrestlessness (impatience)angeracting aggressiveCall your child's doctor right away if your child has any new or worsening emotional symptoms while taking Triptodur.Some people taking GnRH agonists like Triptodur have had seizures. The risk of seizures may be higher in people who:have a history of seizureshave a history of epilepsyhave a history of brain or brain vessel (cerebrovascular) problems or tumorsare taking a medicine that has been connected with seizures such as bupropion or selective serotonin reuptake inhibitors (SSRIs)Seizures have also happened in people who have not had any of these problems. Call your child's doctor right away if your child has a seizure while taking Triptodur. |
|||
| What is Triptodur?Triptodur is an injectable prescription GnRH medicine used for the treatment of children with central precocious puberty (CPP).It is not known if Triptodur is safe and effective in children under 2 years of age. | |||
| Triptodur should not be taken if your child is:allergic to gonadotropin releasing hormone (GnRH), GnRH agonist medicines, or any ingredients in Triptodur. See the end of this Medication Guide for a complete list of ingredients in Triptodur.Some people taking triptorelin, the active ingredient in Triptodur, have had serious allergic reactions. Call your child's doctor or get emergency medical help right away if your child gets any of the following symptoms of a serious allergic reaction: | |||
| skin rashes, redness, or swellingtrouble breathing or swallowingthroat tightness, hoarseness | severe itchingfast heart beatswelling of face, mouth, and tongue | hivessweatingdizziness or fainting | |
| pregnant or becomes pregnant. Triptodur can cause birth defects or loss of the baby. If your child becomes pregnant call your doctor. | |||
| Before your child receives Triptodur, tell your child's doctor about all of your child's medical conditions, including if they:have a history of mental (psychiatric) problems.have a history of seizures.have a history of epilepsy.have a history of brain or brain vessel (cerebrovascular) problems or tumors.are breastfeeding or plan to breastfeed. It is not known if Triptodur passes into breastmilk.Tell the doctor about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |||
| How will your child receive Triptodur?Your child's doctor should do tests to make sure your child has CPP before treating them with Triptodur.Triptodur must only be given by a healthcare professional.Triptodur is given as a single intramuscular (in the muscle) injection 1 time every 24 weeks.Keep all scheduled visits to the doctor. Do not delay a scheduled dose. The doctor will do regular exams and blood tests to check for signs of puberty. | |||
|
What are the possible side effects of Triptodur? Triptodur may cause serious side effects. See "What is the most important information I should know about Triptodur?" The most common side effects of Triptodur include injection site reactions, menstrual (vaginal) bleeding, hot flush, headache, cough, and infections (bronchitis, gastroenteritis, influenza, nasopharyngitis, otitis externa, pharyngitis, sinusitis, and upper respiratory tract infection). These are not all the possible side effects of Triptodur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
What are the ingredients in Triptodur? Active ingredient: triptorelin Inactive ingredients: poly-d,l-lactide-co-glycolide, mannitol, carboxymethylcellulose sodium, and polysorbate 80 Distributed by: Arbor Pharmaceuticals, LLC, Atlanta, GA 30328 Manufactured by: Debiopharm Research & Manufacturing SA, CH-1920 Martigny, Switzerland © 2018 Arbor Pharmaceuticals, LLC All rights reserved. For more information about Triptodur, please contact Arbor Pharmaceuticals, LLC at 1-866-516-4950. |
|||
Triptodur is a registered trademark of Debiopharm International SA.
TRIP-MG-04
NDC 24338-150-20
Rx Only
Triptodur™
(triptorelin)
for extended-release injectable suspension
22.5 mg
KIT
22.5 mg every 24 weeks
FOR INTRAMUSCULAR USE
Give once every 24 weeks.
Single-Dose Delivery System
See package insert for full
prescribing information.
Contents:
–One Single-Dose Vial–One Pre-filled Syringe of Diluent (sterile water)Reconstitute With Accompanying Diluent Before Use.
Dispense the accompanying
Medication Guide to each patient.
arbor
PHARMACEUTICALS, LLC
|
Triptodur triptorelin kit |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - Arbor Pharmaceuticals, LLC (781796417) |