

Rasuvo 甲氨蝶呤




| 通用中文 | 甲氨蝶呤 | 通用外文 | Methotrexate |
| 品牌中文 | 拉苏沃 | 品牌外文 | Rasuvo |
| 其他名称 | |||
| 公司 | MEDAC(MEDAC) | 产地 | 美国(USA) |
| 含量 | 包装 | 1支/盒 | |
| 剂型给药 | 皮下注射 | 储存 | 室温 |
| 适用范围 | 类风湿性关节炎,幼年类风湿性关节炎,银屑病 | ||
| 通用中文 | 甲氨蝶呤 |
| 通用外文 | Methotrexate |
| 品牌中文 | 拉苏沃 |
| 品牌外文 | Rasuvo |
| 其他名称 | |
| 公司 | MEDAC(MEDAC) |
| 产地 | 美国(USA) |
| 含量 | |
| 包装 | 1支/盒 |
| 剂型给药 | 皮下注射 |
| 储存 | 室温 |
| 适用范围 | 类风湿性关节炎,幼年类风湿性关节炎,银屑病 |
Rasuvo (methotrexate) Injection - formerly MPI-2505
Date of Approval: July 11, 2014
Company: Medac Pharma, Inc.
Treatment for: Rheumatoid Arthritis, Juvenile Rheumatoid Arthritis, Psoriasis
Rasuvo (methotrexate) is a subcutaneous, ready-to-use autopen formulation of methotrexate for the treatment of rheumatoid arthritis (RA), poly-articular-course juvenile RA and psoriasis.
芝加哥,IL,2014年7月14日--一家私人控股的制药公司,专注于开发新的分子并提高现有药物的有效性,宣布美国食品和药物管理局已经批准了FDA的批准,皮下注射甲氨蝶呤(mtx)治疗类风湿性关节炎(ra)、多节段幼年特发性关节炎(pjia)和银屑病。Rasuvo将提供10种剂量强度,从7.5毫克到30毫克,增量为2.5毫克,并将在美国上市。
该公司同时宣布,特拉华州地区的美国地方法院驳回了安塔雷斯制药公司提出的初步禁令动议。
“我们很高兴我们的主导产品Rasuvo获得批准,并期待着将这种急需的疗法引入市场,”Medac Pharma,Inc.的总裁兼首席执行官Terri Shoemaker女士说,“甲氨蝶呤长期以来被公认为类风湿关节炎治疗的支柱。由于其几乎无痛给药、广泛的剂量范围和显著提高的生物利用度,我们相信Rasuvo可能有利于那些使用甲氨蝶呤的患者。”关于美国特拉华州地区法院的判决,Shoemaker女士说,“我们对法院的判决感到满意,并正在按计划推进我们的商业化努力。”
经过30多年的临床应用,甲氨蝶呤仍然是治疗类风湿关节炎最常用的药物,被美国风湿病学院和欧洲抗风湿病联盟推荐为治疗类风湿关节炎的一线药物。虽然许多患者口服甲氨蝶呤,但这种给药途径与患者之间的高可变吸收率和不一致的生物利用度有关。
美达克制药公司开发了拉苏沃来优化甲氨蝶呤治疗。Rasuvo的皮下给药方式及其广泛的给药选择,旨在提高生物利用度。
MD西北大学费因伯格医学院医学系的风湿病学家Eric Ruderman说:“作为风湿病专家,我相信Rasuvo会为患者提供机会,使他们从甲氨蝶呤中获益最大化。Rasuvo的给药灵活性,特别是,将是非常有帮助的,因为RA患者并不都对甲氨蝶呤同样反应,因此选择一种适合于患者病情的治疗方案是非常重要的。
有关完整的处方信息,包括盒装警告,请访问www.rasuvo.com。
关于Medac Pharma,Inc.
medac制药有限公司是medac股份有限公司的全资子公司,medac股份有限公司是一家享誉全球的制药公司,已经有40多年的科学和治疗发现。
medac制药公司专注于开发有可能在患者生活中产生有意义的差异的治疗方法。该公司的方法是改进现有的代理商,通过加强交付模式,解决安全概况和发明方法,以最大限度地发挥功效。
资料来源:医药公司。
发布时间:2014年7月
Rasuvo Injection
Rasuvo is indicated in the management of selected adults with severe, active rheumatoid arthritis (RA) (ACR criteria), or children with active polyarticular juvenile idiopathic arthritis (pJIA), who have had an insufficient therapeutic response to, or are intolerant of, an adequate trial of first-line therapy including full dose non-steroidal anti-inflammatory agents (NSAIDs).
PsoriasisRasuvo is indicated in adults for the symptomatic control of severe, recalcitrant, disabling psoriasis that is not adequately responsive to other forms of therapy, but only when the diagnosis has been established, as by biopsy and/or after dermatologic consultation. It is important to ensure that a psoriasis "flare" is not due to an undiagnosed concomitant disease affecting immune responses.
Limitation of UseRasuvo is not indicated for the treatment of neoplastic diseases.
Rasuvo Injection Dosage and AdministrationImportant Dosing InformationRasuvo is a single-dose manually-triggered auto-injector for once-weekly subcutaneous use only [see Warnings and Precautions (5.5)]. Administer Rasuvo in the abdomen or the thigh. Rasuvo is only available in doses between 7.5 to 30 mg in 2.5 mg increments. Use another formulation of methotrexate for alternative dosing in patients who require oral, intramuscular, intravenous, intra-arterial, or intrathecal dosing, doses less than 7.5 mg per week, doses more than 30 mg per week, high-dose regimens, or dose adjustments of less than 2.5 mg increments.
Rheumatoid Arthritis including Polyarticular Juvenile Idiopathic ArthritisRecommended starting dose of methotrexate:
Adult RA: 7.5 mg as a single oral or subcutaneous dose once weekly.
pJIA: 10 mg/m2 once weekly.
For patients switching from oral methotrexate to Rasuvo, consider any differences in bioavailability between oral and subcutaneously administered methotrexate [see Clinical Pharmacology (12.3)].
Dosages may be adjusted gradually to achieve an optimal response. Limited experience shows a significant increase in the incidence and severity of serious toxic reactions, especially bone marrow suppression, at doses greater than 20 mg/wk in adults. Although there is experience with doses up to 30 mg/m2/wk in children, there are too few published data to assess how doses over 20 mg/m2/wk might affect the risk of serious toxicity in children. Experience does suggest, however, that children receiving 20 to 30 mg/m2/wk (0.65 to 1.0 mg/kg/wk) may have better absorption and fewer gastrointestinal side effects if methotrexate is administered either intramuscularly or subcutaneously.
Therapeutic response usually begins within 3 to 6 weeks and the patient may continue to improve for another 12 weeks or more.
The optimal duration of therapy is unknown. Limited data available from long-term studies in adults indicate that the initial clinical improvement is maintained for at least two years with continued therapy. When methotrexate is discontinued, the arthritis usually worsens within 3 to 6 weeks.
The patient should be fully informed of the risks involved and should be under constant supervision of the physician. Assessment of hematologic, hepatic, renal, and pulmonary function should be made by history, physical examination, and laboratory tests before beginning, periodically during, and before reinstituting Rasuvo therapy [see Warnings and Precautions (5.4)]. Females of childbearing potential should not be started on Rasuvo until pregnancy is excluded [see Contraindications (4) and Warnings and Precautions (5.2)].
All schedules should be continually tailored to the individual patient. An initial test dose may be given prior to the regular dosing schedule to detect any extreme sensitivity to adverse effects.
Maximal myelosuppression usually occurs in seven to ten days.
PsoriasisRecommended starting dose of methotrexate:
Psoriasis: 10-25 mg as a single oral, intramuscular, subcutaneous, or intravenous dose once weekly.
For patients switching from oral methotrexate to Rasuvo, consider any differences in bioavailability between oral and subcutaneously administered methotrexate [see Clinical Pharmacology (12.3)].
Dosage may be gradually adjusted to achieve optimal clinical response; 30 mg/week should not ordinarily be exceeded. Once optimal clinical response has been achieved, the dosage should be reduced to the lowest possible amount of drug and to the longest possible rest period. The use of Rasuvo may permit the return to conventional topical therapy, which should be encouraged.
Administration and HandlingRasuvo is a manually-triggered auto-injector intended for subcutaneous use under the guidance and supervision of a physician.
Patients may self-inject with Rasuvo if a physician determines that it is appropriate, if they have received proper training in how to prepare and administer the correct dose, and if they receive medical follow-up, as necessary.
Rasuvo is injected once weekly. The patient must be explicitly informed about the once weekly dosing schedule. It is advisable to determine an appropriate fixed day of the week for the injection.
Visually inspect Rasuvo for particulate matter and discoloration prior to administration. Do not use Rasuvo if the seal is broken.
Handle and dispose of Rasuvo consistent with recommendations for handling and disposal of cytotoxic drugs1.
Dosage Forms and StrengthsRasuvo is an injection containing methotrexate at a concentration of 50 mg/ml available as a manually-triggered auto-injector that administers a single dose of methotrexate solution in the following dosage strengths:
7.5 mg10 mg12.5 mg15 mg17.5 mg20 mg22.5 mg25 mg27.5 mg30 mgContraindicationsRasuvo is contraindicated in the following:
PregnancyRasuvo can cause fetal death or teratogenic effects when administered to a pregnant woman. Rasuvo is contraindicated in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
Nursing MothersBecause of the potential for serious adverse reactions from methotrexate in breast fed infants, Rasuvo is contraindicated in nursing mothers [see Use in Specific Populations (8.3)].
Alcoholism or Liver DiseasePatients with alcoholism, alcoholic liver disease or other chronic liver disease [see Warnings and Precautions (5.1)].
Immunodeficiency SyndromesPatients who have overt or laboratory evidence of immunodeficiency syndromes [see Warnings and Precautions (5.1)].
Preexisting Blood DyscrasiasPatients who have preexisting blood dyscrasias, such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia [see Warnings and Precautions (5.1)].
HypersensitivityPatients with a known hypersensitivity to methotrexate. Severe hypersensitivity reactions have been observed with methotrexate use [see Warnings and Precautions (5.1) and Adverse Reactions (6.1 and 6.2)].
Warnings and PrecautionsOrgan System ToxicityRasuvo should be used only by physicians whose knowledge and experience include the use of antimetabolite therapy. Because of the possibility of serious toxic reactions (which can be fatal), Rasuvo should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease which is not adequately responsive to other forms of therapy.
Deaths have been reported with the use of methotrexate in the treatment of malignancy, psoriasis, and rheumatoid arthritis. Patients should be closely monitored for bone marrow, liver, lung and kidney toxicities.
Rasuvo has the potential for serious toxicity. Toxic effects may be related in frequency and severity to dose or frequency of administration but have been seen at all doses. Because they can occur at any time during therapy, it is necessary to follow patients on Rasuvo closely. Most adverse reactions are reversible if detected early. When such reactions do occur, the drug should be reduced in dosage or discontinued and appropriate corrective measures should be taken. If necessary, this could include the use of leucovorin calcium and/or acute, intermittent hemodialysis with a high-flux dialyzer [see Overdosage (10)]. If Rasuvo therapy is reinstituted, it should be carried out with caution, with adequate consideration of further need for the drug and increased alertness as to possible recurrence of toxicity. The clinical pharmacology of methotrexate has not been well studied in older individuals. Due to diminished hepatic and renal function as well as decreased folate stores in this population, relatively low doses should be considered, and these patients should be closely monitored for early signs of toxicity [see Use in Specific Populations (8.5)].
Gastrointestinal:
Diarrhea and ulcerative stomatitis require interruption of therapy: otherwise, hemorrhagic enteritis and death from intestinal perforation may occur.
If vomiting, diarrhea, or stomatitis occur, which may result in dehydration, Rasuvo should be discontinued until recovery occurs. Rasuvo should be used with extreme caution in the presence of peptic ulcer disease or ulcerative colitis.
Unexpectedly severe (sometimes fatal) gastrointestinal toxicity has been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.1)].
Hematologic:
Rasuvo can suppress hematopoiesis and cause anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, and/or thrombocytopenia. In patients with preexisting hematopoietic impairment, Rasuvo should be used with caution, if at all. In controlled clinical trials conducted with another formulation of methotrexate in rheumatoid arthritis (n=128), leukopenia (WBC <3000/mm3) was seen in 2 patients, thrombocytopenia (platelets <100,000/mm3) in 6 patients, and pancytopenia in 2 patients.
Rasuvo should be stopped immediately if there is a significant drop in blood counts. Patients with profound granulocytopenia and fever should be evaluated immediately and usually require parenteral broad-spectrum antibiotic therapy.
Unexpectedly severe (sometimes fatal) bone marrow suppression and aplastic anemia have been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti- inflammatory drugs (NSAIDs) [see Drug Interactions (7.1)].
Hepatic:
Rasuvo has the potential for acute (elevated transaminases) and chronic (fibrosis and cirrhosis) hepatotoxicity. Chronic toxicity is potentially fatal; it generally has occurred after prolonged use (generally two years or more) and after a total dose of at least 1.5 grams. In studies in psoriatic patients, hepatotoxicity appeared to be a function of total cumulative dose and appeared to be enhanced by alcoholism, obesity, diabetes and advanced age. An accurate incidence rate has not been determined; the rate of progression and reversibility of lesions is not known. Special caution is indicated in the presence of preexisting liver damage or impaired hepatic function.
In psoriasis, liver function tests, including serum albumin, should be performed periodically prior to dosing but are often normal in the face of developing fibrosis or cirrhosis. These lesions may be detectable only by biopsy. The usual recommendation is to obtain a liver biopsy at 1) pretherapy or shortly after initiation of therapy (2 to 4 months), 2) a total cumulative dose of 1.5 grams, and 3) after each additional 1.0 to 1.5 grams. Moderate fibrosis or any cirrhosis normally leads to discontinuation of the drug; mild fibrosis normally suggests a repeat biopsy in 6 months.
Milder histologic findings such as fatty change and low grade portal inflammation are relatively common pretherapy. Although these mild changes are usually not a reason to avoid or discontinue Rasuvo therapy, the drug should be used with caution.
In rheumatoid arthritis, age at first use of methotrexate and duration of therapy have been reported as risk factors for hepatotoxicity; other risk factors, similar to those observed in psoriasis, may be present in rheumatoid arthritis but have not been confirmed to date. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in this population. There is a combined reported experience in 217 rheumatoid arthritis patients with liver biopsies both before and during treatment (after a cumulative dose of at least 1.5 g) and in 714 patients with a biopsy only during treatment. There are 64 (7%) cases of fibrosis and 1 (0.1%) case of cirrhosis. Of the 64 cases of fibrosis, 60 were deemed mild. The reticulin stain is more sensitive for early fibrosis and its use may increase these figures. It is unknown whether even longer use will increase these risks.
Liver function tests should be performed at baseline and at 4 to 8 week intervals in patients receiving Rasuvo for rheumatoid arthritis. Pretreatment liver biopsy should be performed for patients with a history of excessive alcohol consumption, persistently abnormal baseline liver function test values or chronic hepatitis B or C infection. During therapy, liver biopsy should be performed if there are persistent liver function test abnormalities or there is a decrease in serum albumin below the normal range (in the setting of well controlled rheumatoid arthritis).
If the results of a liver biopsy show mild changes (Roenigk, grades I, II, IIIa), Rasuvo may be continued and the patient monitored as per recommendations listed above. Rasuvo should be discontinued in any patient who displays persistently abnormal liver function tests and refuses liver biopsy or in any patient whose liver biopsy shows moderate to severe changes (Roenigk grade IIIb or IV).
Infection or Immunologic States:
Rasuvo should be used with extreme caution in the presence of active infection, and is contraindicated in patients with overt or laboratory evidence of immunodeficiency syndromes.
Immunization may be ineffective when given during Rasuvo therapy. Immunization with live virus vaccines is generally not recommended. There have been reports of disseminated vaccinia infections after smallpox immunizations in patients receiving methotrexate therapy. Hypogammaglobulinemia has been reported rarely.
Potentially fatal opportunistic infections, especially Pneumocystis jiroveci pneumonia, may occur with Rasuvo therapy. When a patient presents with pulmonary symptoms, the possibility of Pneumocystis jiroveci pneumonia should be considered.
Neurologic:
There have been reports of leukoencephalopathy following intravenous administration of methotrexate to patients who have had craniospinal irradiation. Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with acute lymphoblastic leukemia who were treated with intermediate-dose intravenous methotrexate (1 gm/m2). Symptomatic patients were commonly noted to have leukoencephalopathy and/or microangiopathic calcifications on diagnostic imaging studies. Chronic leukoencephalopathy has also been reported in patients who received repeated doses of high-dose methotrexate with leucovorin rescue even without cranial irradiation.
Discontinuation of methotrexate does not always result in complete recovery. A transient acute neurologic syndrome has been observed in patients treated with high dose regimens. Manifestations of this stroke-like encephalopathy may include confusion, hemiparesis, transient blindness, seizures and coma. The exact cause is unknown. After the intrathecal use of methotrexate, the central nervous system toxicity which may occur can be classified as follows: acute chemical arachnoiditis manifested by such symptoms as headache, back pain, nuchal rigidity, and fever; sub-acute myelopathy characterized by paraparesis/paraplegia associated with involvement with one or more spinal nerve roots; chronic leukoencephalopathy manifested by confusion, irritability, somnolence, ataxia, dementia, seizures and coma. This condition can be progressive and even fatal.
Pulmonary:
Methotrexate-induced lung disease, including acute or chronic interstitial pneumonitis, is a potentially dangerous lesion, which may occur acutely at any time during therapy and has been reported at low doses. It is not always fully reversible and fatalities have been reported.
Pulmonary symptoms (especially a dry nonproductive cough) or a non-specific pneumonitis occurring during Rasuvo therapy may be indicative of a potentially dangerous lesion and require interruption of treatment and careful investigation. Although clinically variable, the typical patient with methotrexate induced lung disease presents with fever, cough, dyspnea, hypoxemia, and an infiltrate on chest X-ray; infection (including pneumonia) needs to be excluded. This lesion can occur at all dosages.
Renal:
Rasuvo may cause renal damage that may lead to acute renal failure. High doses of methotrexate used in the treatment of osteosarcoma may cause renal damage leading to acute renal failure. Nephrotoxicity is due primarily to the precipitation of methotrexate and 7-hydroxymethotrexate in the renal tubules. Close attention to renal function including adequate hydration, urine alkalinization and measurement of serum methotrexate and creatinine levels are essential for safe administration.
Skin:
Severe, occasionally fatal, dermatologic reactions, including toxic epidermal necrolysis, Stevens- Johnson syndrome, exfoliative dermatitis, skin necrosis, and erythema multiforme, have been reported in children and adults, within days of oral, intramuscular, intravenous, or intrathecal methotrexate administration. Reactions were noted after single or multiple low, intermediate, or high doses of methotrexate in patients with neoplastic and non-neoplastic diseases.
Lesions of psoriasis may be aggravated by concomitant exposure to ultraviolet radiation.
Radiation dermatitis and sunburn may be "recalled" by the use of methotrexate.
Other precautions:
Rasuvo should be used with extreme caution in the presence of debility.
Methotrexate exits slowly from third space compartments (e.g., pleural effusions or ascites). This results in a prolonged terminal plasma half-life and unexpected toxicity. In patients with significant third space accumulations, it is advisable to evacuate the fluid before treatment and to monitor plasma methotrexate levels.
Embryo-Fetal ToxicityMethotrexate has been reported to cause fetal death and/or congenital anomalies. Therefore, Rasuvo is not recommended for females of childbearing potential unless there is clear medical evidence that the benefits can be expected to outweigh the considered risks. Rasuvo is contraindicated in pregnant women with psoriasis or rheumatoid arthritis.
Females of childbearing potential should not be started on Rasuvo until pregnancy is excluded and should be fully counseled on the serious risk to the fetus should they become pregnant while undergoing treatment. Appropriate steps should be taken to avoid conception during Rasuvo therapy. Pregnancy should be avoided if either partner is receiving Rasuvo; during and for a minimum of three months after therapy for male patients, and during and for at least one ovulatory cycle after therapy for female patients.
Effects on ReproductionMethotrexate has been reported to cause impairment of fertility, oligospermia and menstrual dysfunction in humans, during and for a short period after cessation of therapy.
The risk of effects of reproduction should be discussed with both male and female patients taking Rasuvo.
Laboratory TestsPatients undergoing Rasuvo therapy should be closely monitored so that toxic effects are detected promptly. Baseline assessment should include a complete blood count with differential and platelet counts, hepatic enzymes, renal function tests and a chest X-ray.
During therapy, monitoring of these parameters is recommended: hematology at least monthly, renal function and liver function every 1 to 2 months [see Warnings and Precautions (5.1)].
During initial or changing doses, or during periods of increased risk of elevated methotrexate blood levels (e.g., dehydration), more frequent monitoring may also be indicated.
Liver Function Tests
Transient liver function test abnormalities are observed frequently after methotrexate administration and are usually not cause for modification of methotrexate therapy. Persistent liver function test abnormalities, and/or depression of serum albumin may be indicators of serious liver toxicity and require evaluation [see Warnings and Precautions (5.1)].
A relationship between abnormal liver function tests and fibrosis or cirrhosis of the liver has not been established for patients with psoriasis. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in the rheumatoid arthritis population.
Pulmonary Function Tests
Pulmonary function tests may be useful if methotrexate-induced lung disease is suspected, especially if baseline measurements are available [see Warnings and Precautions (5.1)].
Risks from Improper DosingBoth the physician and pharmacist should emphasize to the patient that Rasuvo is administered once weekly and that mistaken daily use has led to fatal toxicity [see Dosage and Administration (2)].
Patients with Impaired Renal Function, Ascites, or Pleural EffusionsMethotrexate elimination is reduced in patients with impaired renal function, ascites, or pleural effusions. Such patients require especially careful monitoring for toxicity and require dose reduction or, in some cases, discontinuation of Rasuvo administration.
Dizziness and FatigueAdverse reactions, such as dizziness and fatigue, may affect the ability to drive or operate machinery.
Malignant LymphomasNon-Hodgkin's lymphoma and other tumors have been reported in patients receiving low-dose oral methotrexate. However, there have been instances of malignant lymphoma arising during treatment with low-dose oral methotrexate, which have regressed completely following withdrawal of methotrexate, without requiring active anti-lymphoma treatment. Discontinue Rasuvo first and, if the lymphoma does not regress, appropriate treatment should be instituted.
Tumor Lysis SyndromeLike other cytotoxic drugs, methotrexate may induce "tumor lysis syndrome" in patients with rapidly growing tumors.
Concomitant Radiation TherapyMethotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis.
Adverse ReactionsThe following adverse reactions are discussed in more detail in other sections of the labeling.
Organ System Toxicity [see Warnings and Precautions (5.1)]Embryo-Fetal Toxicity [see Warnings and Precautions (5.2)]Effects on Reproduction [see Warnings and Precautions (5.3)]Malignant Lymphomas [see Warnings and Precautions (5.8)]The most frequently reported adverse reactions include ulcerative stomatitis, leukopenia, nausea, and abdominal distress. Other frequently reported adverse reactions are malaise, undue fatigue, chills and fever, dizziness and decreased resistance to infection.
Clinical Trials ExperienceThis section provides a summary of adverse reactions reported in subjects in clinical studies conducted with Rasuvo as well as with methotrexate injection and oral methotrexate.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
Rheumatoid Arthritis
The approximate incidences of methotrexate-attributed (i.e. placebo rate subtracted) adverse reactions in 12 to 18 week double-blind studies of patients (n=128) with rheumatoid arthritis treated with low-dose oral (7.5 to 15 mg/week) pulse methotrexate, are listed below. Virtually all of these patients were on concomitant nonsteroidal anti-inflammatory drugs and some were also taking low dosages of corticosteroids. Hepatic histology was not examined in these short-term studies.
Incidence greater than 10%: Elevated liver function tests 15%, nausea/vomiting 10%.
Incidence 3% to 10%: Stomatitis, thrombocytopenia (platelet count less than 100,000/mm3).
Incidence 1% to 3%: Rash/pruritis/dermatitis, diarrhea, alopecia, leukopenia (WBC less than 3000/mm3), pancytopenia, dizziness.
Two other controlled trials of patients (n=680) with Rheumatoid Arthritis on 7.5 mg to 15 mg/wk oral doses showed an incidence of interstitial pneumonitis of 1%.
Other less common reactions included decreased hematocrit, headache, upper respiratory infection, anorexia, arthralgias, chest pain, coughing, dysuria, eye discomfort, epistaxis, fever, infection, sweating, tinnitus, and vaginal discharge.
Polyarticular Juvenile Idiopathic Arthritis
The approximate incidences of adverse reactions reported in pediatric patients with pJIA treated with oral, weekly doses of methotrexate (5 to 20 mg/m2/wk or 0.1 to 0.65 mg/kg/wk) were as follows (virtually all patients were receiving concomitant nonsteroidal anti-inflammatory drugs, and some also were taking low doses of corticosteroids): elevated liver function tests, 14%; gastrointestinal reactions (e.g., nausea, vomiting, diarrhea), 11%; stomatitis, 2%; leukopenia, 2%; headache, 1.2%; alopecia, 0.5%; dizziness, 0.2%; and rash, 0.2%. Although there is experience with dosing up to 30 mg/m2/wk in pJIA, the published data for doses above 20 mg/m2/wk are too limited to provide reliable estimates of adverse reaction rates.
Psoriasis
There are two literature reports (Roenigk, 1969, and Nyfors, 1978) describing large series (n=204, 248) of psoriasis patients treated with methotrexate. Dosages ranged up to 25 mg per week and treatment was administered for up to four years. With the exception of alopecia, photosensitivity, and "burning of skin lesions" (each 3% to 10%), the adverse reaction rates in these reports were very similar to those in the rheumatoid arthritis studies. Rarely, painful plaque erosions may appear (Pearce, HP and Wilson, BB: Am Acad Dermatol 35: 835-838, 1996).
Other Adverse ReactionsOther adverse reactions that have been reported with methotrexate in oncology, RA, pJIA, and psoriasis patients are listed below by organ system.
Alimentary System: gingivitis, pharyngitis, stomatitis, anorexia, nausea, vomiting, diarrhea, hematemesis, melena, gastrointestinal ulceration and bleeding, enteritis, pancreatitis.
Blood and Lymphatic System Disorders: suppressed hematopoiesis, anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, thrombocytopenia, agranulocytosis, eosinophilia, lymphadenopathy and lymphoproliferative disorders (including reversible). Hypogammaglobulinemia has been reported rarely.
Cardiovascular: pericarditis, pericardial effusion, hypotension, and thromboembolic events (including arterial thrombosis, cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, and pulmonary embolus).
Central Nervous System: headaches, drowsiness, blurred vision, transient blindness, speech impairment including dysarthria and aphasia, hemiparesis, paresis and convulsions have also occurred following administration of methotrexate. Following low doses, there have been occasional reports of transient subtle cognitive dysfunction, mood alteration or unusual cranial sensations, leukoencephalopathy, or encephalopathy.
Hepatobiliary Disorders: hepatotoxicity, acute hepatitis, chronic fibrosis and cirrhosis, hepatic failure, decrease in serum albumin, liver enzyme elevations.
Infection: There have been case reports of sometimes fatal opportunistic infections in patients receiving methotrexate therapy for neoplastic and non-neoplastic diseases. Pneumocystis jiroveci pneumonia was the most common opportunistic infection. There have also been reports of infections, pneumonia, Cytomegalovirus infection, including cytomegaloviral pneumonia, sepsis, fatal sepsis, nocardiosis, histoplasmosis, cryptococcosis, Herpes zoster, Herpes simplex hepatitis, and disseminated Herpes simplex.
Musculoskeletal System: stress fracture.
Ophthalmic: conjunctivitis, serious visual changes of unknown etiology.
Pulmonary System: respiratory fibrosis, respiratory failure, alveolitis, interstitial pneumonitis deaths have been reported, and chronic interstitial obstructive pulmonary disease has occasionally occurred.
Skin: erythematous rashes, pruritus, urticaria, photosensitivity, pigmentary changes, alopecia, ecchymosis, telangiectasia, acne, furunculosis, erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, skin necrosis, skin ulceration and exfoliative dermatitis.
Urogenital System: severe nephropathy or renal failure, azotemia, cystitis, hematuria, proteinuria; defective oogenesis or spermatogenesis, transient oligospermia, menstrual dysfunction, vaginal discharge, and gynecomastia; infertility, abortion, fetal death, fetal defects.
Other rarer reactions related to or attributed to the use of methotrexate such as nodulosis, vasculitis, arthralgia/myalgia, loss of libido/impotence, diabetes, osteoporosis, sudden death, lymphoma, including reversible lymphomas, tumor lysis syndrome, soft tissue necrosis and osteonecrosis. Anaphylactoid reactions have been reported.
Drug InteractionsAspirin, Nonsteroidal Anti-Inflammatory Drugs, and SteroidsNonsteroidal anti-inflammatory drugs (NSAIDs) should not be administered prior to or concomitantly with the high doses of methotrexate, such as used in the treatment of osteosarcoma. Concomitant administration of some NSAIDs with high dose methotrexate therapy has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic and gastrointestinal toxicity [see Warnings and Precautions (5.1)].
Caution should be used when NSAIDs and salicylates are administered concomitantly with lower doses of methotrexate, including Rasuvo. These drugs have been reported to reduce the tubular secretion of methotrexate in an animal model and may enhance its toxicity.
Despite the potential interactions, studies of methotrexate in patients with rheumatoid arthritis have usually included concurrent use of constant dosage regimens of NSAIDs, without apparent problems. It should be appreciated, however, that the doses used in rheumatoid arthritis (7.5 to 15 mg/week) are somewhat lower than those used in psoriasis and that larger doses could lead to unexpected toxicity. Aspirin, NSAIDs, and/or low dose steroids may be continued, although the possibility of increased toxicity with concomitant use of NSAIDs including salicylates has not been fully explored. Steroids may be reduced gradually in patients who respond to methotrexate.
Proton Pump Inhibitors (PPIs) and H2 BlockersUse caution if high-dose methotrexate is administered to patients receiving proton pump inhibitor (PPI) therapy. Case reports and published population pharmacokinetic studies suggest that concomitant use of some PPIs, such as omeprazole, esomeprazole, and pantoprazole, with methotrexate (primarily at high dose), may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. In two of these cases, delayed methotrexate elimination was observed when high-dose methotrexate was co-administered with PPIs, but was not observed when methotrexate was co-administered with ranitidine. However, no formal drug interaction studies of methotrexate with ranitidine have been conducted.
Oral AntibioticsOral antibiotics such as tetracycline, chloramphenicol, and nonabsorbable broad spectrum antibiotics, may decrease intestinal absorption of methotrexate or interfere with the enterohepatic circulation by inhibiting bowel flora and suppressing metabolism of the drug by bacteria.
Penicillins may reduce the renal clearance of methotrexate; increased serum concentrations of methotrexate with concomitant hematologic and gastrointestinal toxicity have been observed with high and low dose methotrexate. Use of Rasuvo with penicillins should be carefully monitored.
Trimethoprim/sulfamethoxazole has been reported rarely to increase bone marrow suppression in patients receiving methotrexate, probably by decreased tubular secretion and/or an additive antifolate effect.
HepatotoxinsThe potential for increased hepatotoxicity when methotrexate is administered with other hepatotoxic agents has not been evaluated. However, hepatotoxicity has been reported in such cases. Therefore, patients receiving concomitant therapy with Rasuvo and other potential hepatotoxins (e.g., azathioprine, retinoids, and sulfasalazine) should be closely monitored for possible increased risk of hepatotoxicity.
TheophyllineMethotrexate may decrease the clearance of theophylline; theophylline levels should be monitored when used concurrently with Rasuvo.
Folic Acid and AntifolatesVitamin preparations containing folic acid or its derivatives may decrease responses to systemically administered methotrexate. Preliminary animal and human studies have shown that small quantities of intravenously administered leucovorin enter the CSF primarily as 5-methyltetrahydrofolate and, in humans, remain 1 to 3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration. However, high doses of leucovorin may reduce the efficacy of intrathecally administered methotrexate. Folate deficiency states may increase methotrexate toxicity.
Trimethoprim/sulfamethoxazole has been reported rarely to increase bone marrow suppression in patients receiving methotrexate, probably by decreased tubular secretion and/or an additive antifolate effect.
MercaptopurineMethotrexate increases the plasma levels of mercaptopurine. The combination of Rasuvo and mercaptopurine may therefore require dose adjustment.
Nitrous oxideThe use of nitrous oxide anesthesia potentiates the effect of methotrexate on folate dependent metabolic pathways, resulting in the potential for increased toxicity. Avoid the simultaneous use of nitrous oxide and methotrexate.
Other DrugsMethotrexate is partially bound to serum albumin, and toxicity may be increased because of displacement by certain drugs, such as salicylates, phenylbutazone, phenytoin, and sulfonamides.
Renal tubular transport is also diminished by probenecid; use of Rasuvo with this drug should be carefully monitored.
Combined use of methotrexate with gold, penicillamine, hydroxychloroquine, sulfasalazine, or cytotoxic agents, has not been studied and may increase the incidence of adverse effects.
USE IN SPECIFIC POPULATIONSPregnancyPregnancy Category X [see Contraindications (4)]
Methotrexate has been reported to cause embryotoxicity, fetal death, congenital anomalies, and abortion in humans and is contraindicated in pregnant women.
Nursing MothersBecause of the potential for serious adverse reactions from methotrexate in breast fed infants, methotrexate is contraindicated in nursing mothers. Therefore, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Methotrexate has been detected in human breast milk. The highest breast milk to plasma concentration ratio reached was 0.08:1.
Pediatric UseThe safety and effectiveness of methotrexate, including Rasuvo, have not been established in pediatric patients with psoriasis.
The safety and effectiveness of Rasuvo have not been established in pediatric patients with neoplastic diseases.
The safety and effectiveness of methotrexate have been established in pediatric patients with polyarticular juvenile idiopathic arthritis [see Clinical Studies (14.2)].
Published clinical studies evaluating the use of methotrexate in children and adolescents (i.e., patients 2 to 16 years of age) with pJIA demonstrated safety comparable to that observed in adults with rheumatoid arthritis [see Adverse Reactions (6.1)].
Rasuvo does not contain a preservative. However, methotrexate injectable formulations containing the preservative benzyl alcohol are not recommended for use in neonates. There have been reports of fatal 'gasping syndrome' in neonates (children less than one month of age) following the administrations of intravenous solutions containing the preservative benzyl alcohol. Symptoms include a striking onset of gasping respiration, hypotension, bradycardia, and cardiovascular collapse.
Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with acute lymphoblastic leukemia who were treated with intermediate-dose intravenous methotrexate (1 gm/m2) [see Warnings and Precautions (5.1)].
Geriatric UseClinical studies of methotrexate did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic and renal function, decreased folate stores, concomitant disease or other drug therapy (i.e., that interfere with renal function, methotrexate or folate metabolism) in this population [see Warnings and Precautions (5.1), Drug Interactions (7.7) and Use in Specific Populations (8.7)]. Since decline in renal function may be associated with increases in adverse reactions and serum creatinine measurements may over estimate renal function in the elderly, more accurate methods (i.e., creatinine clearance) should be considered. Serum methotrexate levels may also be helpful. Elderly patients should be closely monitored for early signs of hepatic, bone marrow and renal toxicity. In chronic use situations, certain toxicities may be reduced by folate supplementation.
Post-marketing experience suggests that the occurrence of bone marrow suppression, thrombocytopenia, and pneumonitis may increase with age [see Warnings and Precautions (5.1)].
Females and Males of Reproductive PotentialRasuvo is not recommended for females of childbearing potential unless there is clear medical evidence that the benefits can be expected to outweigh the considered risks. Females of childbearing potential should not be started on methotrexate until pregnancy is excluded and should be fully counseled on the serious risk to the fetus should they become pregnant while undergoing treatment [see Use in Specific Populations (8.1)].
Appropriate steps should be taken to avoid conception during Rasuvo therapy. Pregnancy should be avoided if either partner is receiving methotrexate; during and for a minimum of three months after therapy for male patients, and during and for at least one ovulatory cycle after therapy for female patients.
Methotrexate has been reported to cause impairment of fertility, oligospermia and menstrual dysfunction in humans, during and for a short period after cessation of therapy.
Renal ImpairmentMethotrexate elimination is reduced in patients with impaired renal function. Such patients require especially careful monitoring for toxicity and require dose reduction or, in some cases, discontinuation of Rasuvo administration.
Hepatic ImpairmentThe effect of hepatic impairment on methotrexate pharmacokinetics has not been studied. Rasuvo is contraindicated in patients with alcoholic liver disease or other chronic liver disease. Patients with obesity, diabetes, hepatic fibrosis or steatohepatitis are at increased risk for hepatic injury and fibrosis secondary to methotrexate, and should be monitored closely [see Warnings and Precautions (5.1)].
OverdosageLeucovorin is indicated to diminish the toxicity and counteract the effect of inadvertently administered overdosages of methotrexate. Leucovorin administration should begin as promptly as possible. As the time interval between methotrexate administration and leucovorin initiation increases, the effectiveness of leucovorin in counteracting toxicity decreases. Monitoring of the serum methotrexate concentration is essential in determining the optimal dose and duration of treatment with leucovorin.
In cases of massive overdosage, hydration and urinary alkalinization may be necessary to prevent the precipitation of methotrexate and/or its metabolites in the renal tubules. Generally speaking, neither hemodialysis nor peritoneal dialysis has been shown to improve methotrexate elimination. However, effective clearance of methotrexate has been reported with acute, intermittent hemodialysis using a high-flux dialyzer (Wall, SM et al: Am J Kidney Dis 28 (6): 846-854, 1996).
Accidental intrathecal overdosage may require intensive systemic support, high-dose systemic leucovorin, alkaline diuresis and rapid CSF drainage and ventriculolumbar perfusion.
In postmarketing experience, overdose with methotrexate has generally occurred with oral and intrathecal administration, although intravenous and intramuscular overdose have also been reported.
Reports of oral overdose often indicate accidental daily administration instead of weekly (single or divided doses). Symptoms commonly reported following oral overdose include those symptoms and signs reported at pharmacologic doses, particularly hematologic and gastrointestinal reaction. For example, leukopenia, thrombocytopenia, anemia, pancytopenia, bone marrow suppression, mucositis, stomatitis, oral ulceration, nausea, vomiting, gastrointestinal ulceration, gastrointestinal bleeding. In some cases, no symptoms were reported.
There have been reports of death following overdose. In these cases, events such as sepsis or septic shock, renal failure, and aplastic anemia were also reported.
Symptoms of intrathecal overdose are generally central nervous system (CNS) symptoms, including headache, nausea and vomiting, seizure or convulsion, and acute toxic encephalopathy. In some cases, no symptoms were reported. There have been reports of death following intrathecal overdose. In these cases, cerebellar herniation associated with increased intracranial pressure, and acute toxic encephalopathy have also been reported.
There are published case reports of intravenous and intrathecal carboxypeptidase G2 treatment to hasten clearance of methotrexate in cases of overdose.
Rasuvo Injection DescriptionRasuvo contains methotrexate, a folate analog metabolic inhibitor.
Chemically, methotrexate is [N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-Lglutamic acid. The structural formula is:
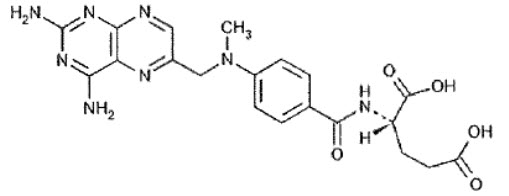
C20H22N8O5 M.W.= 454.45
Rasuvo contains methotrexate in a sterile, preservative-free, non-pyrogenic solution for a single subcutaneous injection. Rasuvo is an isotonic, clear, yellow to brown solution.
Rasuvo contains the following inactive ingredients: sodium chloride 0.4% w/v; water for injections, sodium hydroxide and, if necessary, hydrochloric acid are added to adjust the pH to approximately 8.5.
Rasuvo Injection - Clinical PharmacologyMechanism of ActionMethotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. Therefore, methotrexate interferes with DNA synthesis, repair, and cellular replication. Actively proliferating tissues such as malignant cells, bone marrow, fetal cells, buccal and intestinal mucosa, and cells of the urinary bladder are in general more sensitive to this effect of methotrexate.
The mechanism of action in rheumatoid arthritis is unknown; it may affect immune function.
PharmacodynamicsTwo reports describe in vitro methotrexate inhibition of DNA precursor uptake by stimulated mononuclear cells, and another describes in animal polyarthritis partial correction by methotrexate of spleen cell hyporesponsiveness and suppressed IL 2 production. Other laboratories, however, have been unable to demonstrate similar effects. Clarification of methotrexate's effect on immune activity and its relation to rheumatoid immunopathogenesis await further studies.
In psoriasis, the rate of production of epithelial cells in the skin is greatly increased over normal skin. This differential in proliferation rates is the basis for the use of methotrexate to control the psoriatic process.
Methotrexate in high doses, followed by leucovorin rescue, is used as a part of the treatment of patients with non- metastatic osteosarcoma. The original rationale for high dose methotrexate therapy was based on the concept of selective rescue of normal tissues by leucovorin. More recent evidence suggests that high dose methotrexate may also overcome methotrexate resistance caused by impaired active transport, decreased affinity of dihydrofolic acid reductase for methotrexate, increased levels of dihydrofolic acid reductase resulting from gene amplification, or decreased polyglutamation of methotrexate. The actual mechanism of action is unknown.
PharmacokineticsAbsorption
In adults, oral absorption appears to be dose dependent. Peak serum levels are reached within one to two hours. At doses of 30 mg/m2 or less, methotrexate is generally well absorbed with a mean bioavailability of about 60%. The absorption of doses greater than 80 mg/m2 is significantly less, possibly due to a saturation effect.
In a relative bioavailability study in healthy subjects, the systemic exposure of methotrexate (AUC) from Rasuvo at doses of 7.5 mg, 15 mg, 22.5 mg, and 30 mg, was higher than that of oral methotrexate administered at the same doses by 35%, 49%, 51%, and 68%, respectively. In a relative bioavailability study in psoriasis patients, the systemic exposure (AUC) of methotrexate from Rasuvo at a dose of 30 mg, was similar to that of methotrexate administered at the same dose by the intramuscular route.
In leukemic pediatric patients, oral absorption of methotrexate also appears to be dose dependent and has been reported to vary widely (23% to 95%). A twenty fold difference between highest and lowest peak levels (Cmax: 0.11 to 2.3 micromolar after a 20 mg/m2 dose) has been reported.
Significant interindividual variability has also been noted in time to peak concentration (Tmax: 0.67 to 4 hrs after a 15 mg/m2 dose) and fraction of dose absorbed. The absorption of doses greater than 40 mg/m2 has been reported to be significantly less than that of lower doses. Food has been shown to delay absorption and reduce peak concentration.
Methotrexate is generally completely absorbed from parenteral routes of injection. After intramuscular injection, peak serum concentrations occur in 30 to 60 minutes.
As in leukemic pediatric patients, a wide interindividual variability in the plasma concentrations of methotrexate has been reported in pediatric patients with JIA. Following oral administration of methotrexate in doses of 6.4 to 11.2 mg/m2/week in pediatric patients with JIA, mean serum concentrations were 0.59 micromolar (range, 0.03 to 1.40) at 1 hour, 0.44 micromolar (range, 0.01 to 1.00) at 2 hours, and 0.29 micromolar (range, 0.06 to 0.58) at 3 hours.
Distribution
After intravenous administration, the initial volume of distribution is approximately 0.18 L/kg (18% of body weight) and steady-state volume of distribution is approximately 0.4 to 0.8 L/kg (40 to 80% of body weight). Methotrexate competes with reduced folates for active transport across cell membranes by means of a single carrier-mediated active transport process. At serum concentrations greater than 100 micromolar, passive diffusion becomes a major pathway by which effective intracellular concentrations can be achieved. Methotrexate in serum is approximately 50% protein bound. Laboratory studies demonstrate that it may be displaced from plasma albumin by various compounds including sulfonamides, salicylates, tetracyclines, chloramphenicol, and phenytoin.
Methotrexate does not penetrate the blood-cerebrospinal fluid barrier in therapeutic amounts when given orally or parenterally. High CSF concentrations of the drug may be attained by intrathecal administration of other parenteral forms of methotrexate.
In dogs, synovial fluid concentrations after oral dosing were higher in inflamed than uninflamed joints. Although salicylates did not interfere with this penetration, prior prednisone treatment reduced penetration into inflamed joints to the level of normal joints.
Metabolism
After absorption, methotrexate undergoes hepatic and intracellular metabolism to polyglutamated forms which can be converted back to methotrexate by hydrolase enzymes. These polyglutamates act as inhibitors of dihydrofolate reductase and thymidylate synthetase. Small amounts of methotrexate polyglutamates may remain in tissues for extended periods. The retention and prolonged drug action of these active metabolites vary among different cells, tissues and tumors. A small amount of metabolism to 7-hydroxymethotrexate may occur at doses commonly prescribed. Accumulation of this metabolite may become significant at the high doses used in osteogenic sarcoma. The aqueous solubility of 7-hydroxymethotrexate is 3 to 5 fold lower than the parent compound. Methotrexate is partially metabolized by intestinal flora after oral administration.
Half-Life
The terminal half-life reported for methotrexate is approximately three to ten hours for patients receiving treatment for psoriasis, or rheumatoid arthritis or low dose antineoplastic therapy (less than 30 mg/m2). For patients receiving high doses of methotrexate, the terminal half-life is eight to 15 hours.
In pediatric patients receiving methotrexate for acute lymphocytic leukemia (6.3 to 30 mg/m2), or for JIA (3.75 to 26.2 mg/m2), the terminal half-life has been reported to range from 0.7 to 5.8 hours or 0.9 to 2.3 hours, respectively.
Excretion
Renal excretion is the primary route of elimination and is dependent upon dosage and route of administration. With IV administration, 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. There is limited biliary excretion amounting to 10% or less of the administered dose. Enterohepatic recirculation of methotrexate has been proposed.
Renal excretion occurs by glomerular filtration and active tubular secretion. Nonlinear elimination due to saturation of renal tubular reabsorption has been observed in psoriatic patients at doses between 7.5 and 30 mg. Impaired renal function, as well as concurrent use of drugs such as weak organic acids that also undergo tubular secretion, can markedly increase methotrexate serum levels.
Excellent correlation has been reported between methotrexate clearance and endogenous creatinine clearance.
Methotrexate clearance rates vary widely and are generally decreased at higher doses. Delayed drug clearance has been identified as one of the major factors responsible for methotrexate toxicity. It has been postulated that the toxicity of methotrexate for normal tissues is more dependent upon the duration of exposure to the drug rather than the peak level achieved. When a patient has delayed drug elimination due to compromised renal function, a third space effusion, or other causes, methotrexate serum concentrations may remain elevated for prolonged periods.
When other forms of parenteral methotrexate are administered during cancer chemotherapy, the potential for toxicity from high dose regimens or delayed excretion is reduced by the administration of leucovorin calcium during the final phase of methotrexate plasma elimination.
Pharmacokinetic monitoring of methotrexate serum concentrations may help identify those patients at high risk for methotrexate toxicity and aid in proper adjustments of leucovorin dosing.
Nonclinical ToxicologyCarcinogenesis, Mutagenesis, Impairment of FertilityMethotrexate has been evaluated in a number of animal studies for carcinogenic potential with inconclusive results. Although there is evidence that methotrexate causes chromosomal damage to animal somatic cells and human bone marrow cells, the clinical significance remains uncertain.
Data are available regarding the risks for pregnancy and for fertility in humans [see Use in Specific Populations (8.1 and 8.6)].
Clinical StudiesRheumatoid ArthritisClinical trials in patients with rheumatoid arthritis were performed using other formulations of methotrexate.
In patients with rheumatoid arthritis, effects of methotrexate on articular swelling and tenderness can be seen as early as 3 to 6 weeks.
Most studies of methotrexate in patients with rheumatoid arthritis are relatively short term (3 to 6 months). Limited data from long-term studies indicate that an initial clinical improvement is maintained for at least two years with continued therapy.
Polyarticular Juvenile Idiopathic ArthritisClinical trials in patients with polyarticular juvenile idiopathic arthritis were performed using other formulations of methotrexate.
In a 6-month double-blind, placebo-controlled trial of 127 pediatric patients with pJIA (mean age, 10.1 years; age range, 2.5 to 18 years; mean duration of disease, 5.1 years) on background nonsteroidal anti-inflammatory drugs and/or prednisone, methotrexate given weekly at an oral dose of 10 mg/m2 provided significant clinical improvement compared to placebo as measured by either the physician's global assessment, or by a patient composite (25% reduction in the articular-severity score plus improvement in parent and physician global assessments of disease activity). Over two-thirds of the patients in this trial had polyarticular-course JIA, and the numerically greatest response was seen in this subgroup treated with 10 mg/m2/wk methotrexate.
The overwhelming majority of the remaining patients had systemic-course JIA. All patients were unresponsive to NSAIDs; approximately one-third were using low dose corticosteroids.
Weekly methotrexate at a dose of 5 mg/m2 was not significantly more effective than placebo in this trial.
REFERENCES1. "Hazardous Drugs". OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
How Supplied/Storage and HandlingRasuvo contains methotrexate in a preservative-free sterile solution for a single subcutaneous injection in the following configurations.
| Strength | Pack Configuration* | NDC |
|---|---|---|
| *Single unit configurations are not for sale. Sample only. | ||
| 7.5 mg per 0.15 mL | 1 | 59137-505-01 |
| 4 | 59137-505-04 | |
| 10 mg per 0.20 mL | 1 | 59137-510-01 |
| 4 | 59137-510-04 | |
| 12.5 mg per 0.25 mL | 1 | 59137-515-01 |
| 4 | 59137-515-04 | |
| 15 mg per 0.30 mL | 1 | 59137-520-01 |
| 4 | 59137-520-04 | |
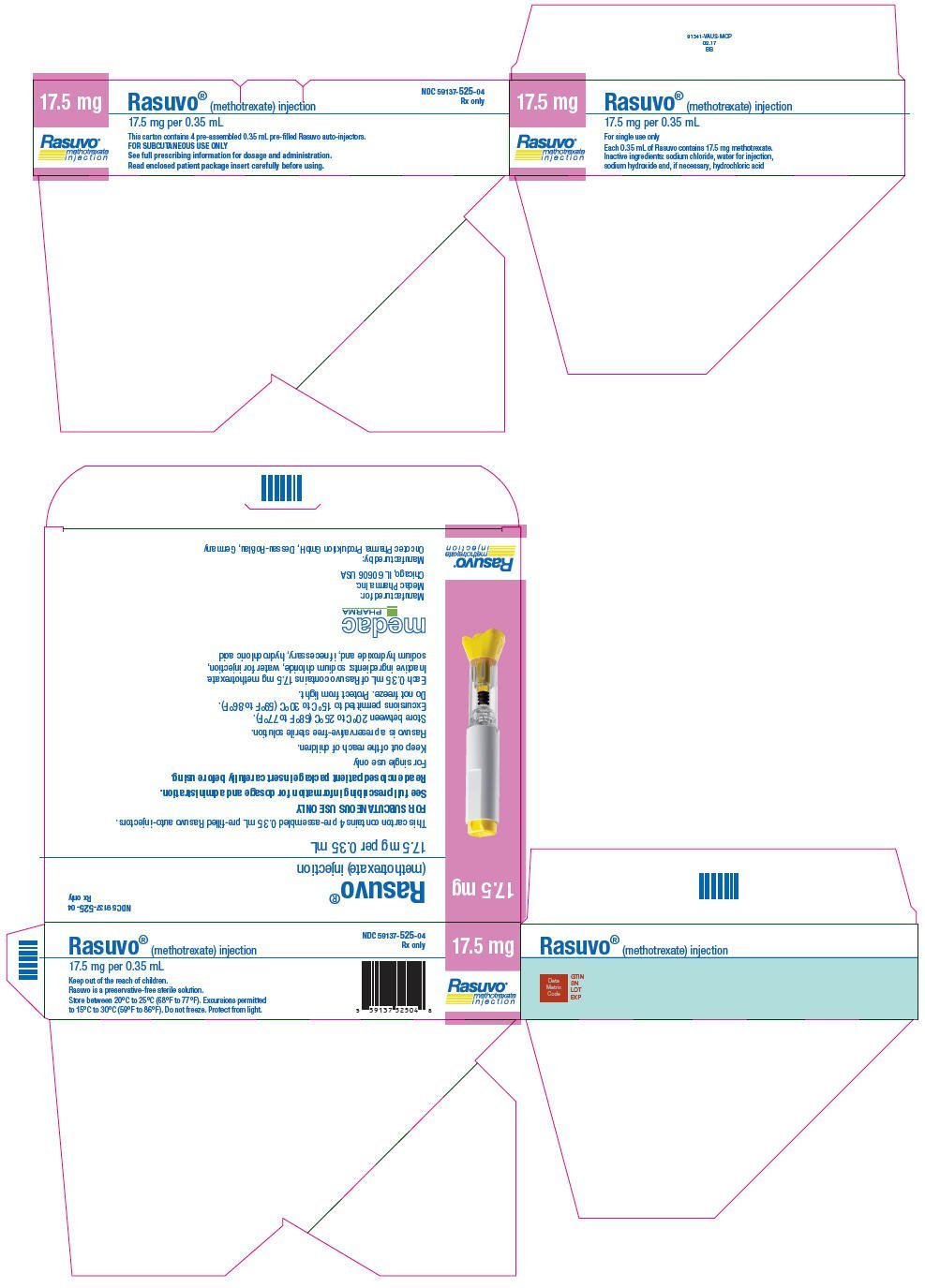
| 17.5 mg per 0.35 mL | 1 | 59137-525-01 |
| 4 | 59137-525-04 | |
| 20 mg per 0.40 mL | 1 | 59137-530-01 |
| 4 | 59137-530-04 | |
| 22.5 mg per 0.45 mL | 1 | 59137-535-01 |
| 4 | 59137-535-04 | |
| 25 mg per 0.50 mL | 1 | 59137-540-01 |
| 4 | 59137-540-04 | |
| 27.5 mg per 0.55 mL | 1 | 59137-545-01 |
| 4 | 59137-545-04 | |
| 30 mg per 0.60 mL | 1 | 59137-550-01 |
| 4 | 59137-550-04 | |
Not all pack sizes may be marketed.
Store at controlled room temperature, 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). PROTECT FROM LIGHT.
Handling and Disposal
Handle and dispose of Rasuvo consistent with recommendations for handling and disposal of cytotoxic drugs.1
Patient Counseling InformationSee FDA-approved patient labeling (Patient Information and Instructions for Use)
Risk of Organ Toxicity
Inform patients of the risks of organ toxicity, including gastrointestinal, hematologic, hepatic, infections, neurologic, pulmonary, renal and skin as well as possible signs and symptoms for which they should contact their healthcare provider. Advise patients of the need for close follow-up, including periodic laboratory tests to monitor toxicity [see Warnings and Precautions (5.1 and 5.4)].
Importance of Proper Dosing and Administration
Both the physician and pharmacist should emphasize to the patient that the recommended dose is taken once weekly and that mistaken daily use of the recommended dose has led to fatal toxicity [see Dosage and Administration (2)].
Rasuvo is intended for use under the guidance and supervision of a physician. Patients should not self-administer until they receive training from a healthcare professional. The patient's or caregiver's ability to administer Rasuvo should be assessed.
Patients should be instructed to use administration sites on the abdomen or the thigh. Administration should not be made within 2 inches of the navel. Instruct patients not to administer Rasuvo to the arms or any other areas of the body, as delineated in the Rasuvo Instructions for Use [see Instructions for Use].
Risks of Pregnancy and Reproduction
Advise patients that Rasuvo can cause fetal harm and is contraindicated in pregnancy. Advise women of childbearing potential that Rasuvo should not be started until pregnancy is excluded. Women should be fully counseled on the serious risk to the fetus should they become pregnant while undergoing treatment. Inform patients to contact their physician if they suspect that they are pregnant.
Advise patients that pregnancy should be avoided if either partner is receiving Rasuvo; during and for a minimum of three months after therapy for male patients, and during and for at least one ovulatory cycle after therapy for female patients [see Warnings and Precautions (5.2)].
Discuss the risk of effects on reproduction with both male and female patients taking Rasuvo.
Inform patients that methotrexate has been reported to cause impairment of fertility, oligospermia and menstrual dysfunction, during and for a short period after cessation of therapy [see Use in Specific Populations (8.6)].
Nursing Mothers
Inform patients that Rasuvo is contraindicated in nursing mothers [see Use in Specific Populations (8.3)].
Ability to Drive or Operate Machinery
Inform patients that adverse reactions such as dizziness and fatigue may affect their ability to drive or operate machinery.
Proper Storage and Disposal
Advise patients to store Rasuvo at room temperature (68 to 77°F or 20 to 25°C). Inform patients and caregivers of the need for proper disposal after use, including the use of a sharps disposal container.
Manufactured for:
Medac Pharma Inc.
29 N Wacker Drive, Suite 704
Chicago, IL 60606
Manufactured by:
Oncotec Pharma Produktion GmbH
Am Pharmapark
D-06861 Dessau-Roßlau
Germany
RASUVO® (ruh-SOO-voh)
(methotrexate)
injection, for subcutaneous use
What is Rasuvo?
Rasuvo is a single-dose manually-triggered auto-injector containing a prescription medicine, methotrexate. Methotrexate is used to:
treat certain adults with severe, active rheumatoid arthritis (RA), and children with active polyarticular juvenile idiopathic arthritis (pJIA), after treatment with other medicines including non-steroidal anti-inflammatory (NSAIDs) have been used and did not work well.control the symptoms of severe, resistant, disabling psoriasis in adults when other types of treatment have been used and did not work well.Rasuvo is available in doses of 7.5, 10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5 and 30 mg. Your doctor will prescribe a different way to take methotrexate if you need to take methotrexate by mouth or in some other way. Your doctor may also change your prescription if your dose does not match the available Rasuvo doses, such as doses of less than 7.5 mg, more than 30 mg, or doses in between the available Rasuvo doses.
Rasuvo should not be used for the treatment of cancer.
Rasuvo should not be used for the treatment of children with psoriasis.
What is the most important information I should know about Rasuvo?
Rasuvo can cause serious side effects that can lead to death, including:
1.Organ system toxicity. People who use methotrexate for the treatment of cancer, psoriasis, or rheumatoid arthritis, have an increased risk of death from organ toxicity. Types of organ toxicity can include:| gastrointestinalbone marrowliverimmune system | nervelungkidneysskin |
| vomitingdiarrheamouth soresfeverconfusionweaknesstemporary blindnessseizuresheadacheback pain | neck stiffnessparalysisirritabilitysleepinessproblems with coordinationdry coughtrouble breathingsevere skin rashinfection |
Who should not take Rasuvo?
Do not take Rasuvo if you:
are pregnant or planning to become pregnant. See "What is the most important information I should know about Rasuvo?"are breastfeeding.Rasuvo can pass into your breast milk and may harm your baby. Do not breastfeed while taking Rasuvo. Talk to your doctor about the best way to feed your baby if you take Rasuvo.have alcohol problems (alcoholism)have liver problemshave problems fighting infection (immunodeficiency syndrome)have been told you have (or think you have) a blood disorder such as low levels of white blood cells, red blood cells (anemia), or platelets.have had an allergy to methotrexate or any of the ingredients in Rasuvo. See the end of this leaflet for a complete list of ingredients in Rasuvo.Talk to your doctor before taking this medicine if you have any of these conditions.
What should I tell my doctor before taking Rasuvo?
Before you take Rasuvo, tell your doctor if you have any other medical conditions.
Tell your doctor about all of the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements.
Rasuvo may affect how other medicines work, and other medicines may affect how Rasuvo works causing side effects.
Ask your doctor or pharmacist for a list of medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Rasuvo?
Read the Instructions for Use that come with Rasuvo.Take Rasuvo exactly as your doctor tells you to take it.Inject Rasuvo only 1 time each week. Do not take Rasuvo every day.Taking Rasuvo every day may cause death from toxicity.Your doctor will show you or your caregiver how to inject Rasuvo. You should not inject Rasuvo until you have been trained on the right way to use it.Check Rasuvo before you inject it. Rasuvo should be yellow to brown in color and should not have any lumps or particles in it.Rasuvo should be injected under the skin of the abdomen or thigh.Do not inject Rasuvo within 2 inches of the belly button (navel)Use a different site each time you inject. This may help to decrease any reactions at the injection site.Do not inject Rasuvo in the arms or any other areas of the body.Do not inject Rasuvo in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.If you are not sure if Rasuvo was injected, or if you have hard time giving the injection, do not inject another dose. Call your pharmacist or doctor right away.If you inject too much Rasuvo, call your doctor or go to the nearest hospital emergency room right away.What should I avoid while taking Rasuvo?
Do not drink alcohol while taking Rasuvo. Drinking alcohol can increase your chances of getting serious side effects.Rasuvo can cause dizziness and tiredness. Do not drive a car, operate machinery, or do anything that needs you to be alert until you know how Rasuvo affects you.Certain vaccinations should be avoided while taking Rasuvo. Talk to your doctor before you or members of your household receive any vaccines.What are the possible side effects of Rasuvo?
Rasuvo may cause serious side effects, including:
See "What is the most important information I should know about Rasuvo?"
fertility problems. Methotrexate, the active ingredient in Rasuvo, may affect your ability to have a baby. Males may have a decreased sperm count, and females may have changes to their menstrual cycle. This can happen while taking Rasuvo and for a short period of time after you stop.certain cancers. Some people who have taken methotrexate have had a certain type of cancer called Non-Hodgkin's lymphoma and other tumors. Your doctor may tell you to stop taking Rasuvo if this happens.tissue and bone problems. Taking methotrexate while having radiation therapy may increase the risk of your tissue or bone not receiving enough blood. This may lead to death of the tissue or bone.Common side effects of Rasuvo include:
| nauseastomach painindigestion (dyspepsia)mouth soresrashstuffy or runny nose and sore throatdiarrheaabnormal liver function testsvomiting | headachebronchitislow red, white, and platelet blood cell counthair lossdizzinesssensitivity to lightburning skin lesionslung problems |
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Rasuvo. For more information, ask your doctor or pharmacist.
Call you doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I dispose of Rasuvo?
Do not throw away in the household trash. Put used Rasuvo in an FDA-cleared sharps disposal container right away after use.If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:made of a heavy-duty plasticcan be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come outupright stable during useleak-resistantproperly labeled to warn of hazardous waste inside the containerWhen your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.Safely dispose of Rasuvo that is out of date or is no longer needed.How should I store Rasuvo?
Store Rasuvo at room temperature between 68°F to 77°F (20°C to 25°C)
Do not freezeKeep Rasuvo out of the light.Keep Rasuvo and all medicines out of the reach of children.
General information about the safe and effective use of Rasuvo.
Methotrexate is sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Rasuvo for a condition for which it was not prescribed. Do not give Rasuvo to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Rasuvo. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about Rasuvo that is written for health professionals.
For more information, please contact Medac Pharma, Inc. at our number 1-855-336-3322.
What are the ingredients in Rasuvo?
Active ingredient: methotrexate
Inactive ingredients: sodium chloride, sodium hydroxide and water for injection, USP, and if necessary hydrochloric acid, USP.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Medac Pharma, Inc.
29 N Wacker Drive, Suite 704
Chicago, IL 60606
Issued: 11/2014
Read this Instructions for Use before using
Rasuvo® (ruh-SOO-voh) (methotrexate) injection, for subcutaneous use
Follow these instructions each time you use Rasuvo®.
| Parts of your Rasuvo auto-injector | |
|---|---|
|
(Figure A) |
|
|
(Figure B) |
|
|
a) Pre-filled auto-injector with cap before injection b) Pre-filled auto-injector after cap removal before injection c) Pre-filled auto-injector after injection |
|
Prepare to Use Rasuvo
Wash your hands well with soap and warm water.Select a clean, well-lit, flat work surface, such as a table.Place the Rasuvo carton containing the auto-injector on your flat work surface.Be sure that the dose, either 7.5, 10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5 or 30 mg, stated on the carton is the same as the dose prescribed by your doctor.Check the expiration date on the label. Do not use if expired.Remove one Rasuvo auto-injector from the packaging.If the Rasuvo appears to be damaged do not use it. Use another Rasuvo.In addition to Rasuvo, you will need the following items: one alcohol swab and one cotton ball or gauze and small adhesive bandage strip, if desired.Check the Liquid
Look at the transparent control zone (see Figure C). The prefilled syringe is visible within the transparent control zone. Examine the contents of the syringe carefully. If the syringe is cracked or broken, do not use it. Use another auto-injector.The liquid should be clear and yellow to brown in color and should not have any lumps or particles in it. Do not use Rasuvo if the liquid is cloudy, discolored or contains particles.You may see an air bubble. This is normal.If you are not able to see or to check the Rasuvo auto-injector correctly prior to injection, ask a caretaker for assistance.Do not remove the yellow cap from the auto-injector until you are ready to use Rasuvo.Choose an Injection Site
Rasuvo should be injected into the stomach (abdomen) or the upper thigh. Do not inject Rasuvo within 2 inches of the belly button (navel) (see Figure D).Do not inject Rasuvo in the arms or any other areas of the body.Do not inject Rasuvo in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.Use a different site each time you inject. This may help to decrease any reactions at the injection site.Wipe the area with an alcohol swab (see Figure E).Allow the skin to dry and do not touch this area again before giving Rasuvo. Do not fan or blow on the clean area.Give your Injection
STEP 1: Remove the Yellow Cap (see Figure F)
Hold the Rasuvo auto-injector with one hand in the handling area.Use your other hand to pull the yellow cap straight off (see Figure F). Do not twist the cap. If you are unable to remove the cap, ask a caretaker for assistance.You may notice 1 or 2 drops of medicine. This is normal.Caution!
Do not touch the needle end with your hands or fingers.This could inject the medicine into your hand.To avoid any injury, never insert your fingers in the opening of the protect tube covering the needle.Do not replace the cap after it has been removed.After the cap is removed Rasuvo must be used without delay or disposed of safely.Do not press the yellow injection button until you are ready to inject Rasuvo.STEP 2: Prepare the Injection
Pinch a pad of skin surrounding the cleaned injection site with your thumb and forefinger of your free hand by gently squeezing. Patients with rheumatoid arthritis who are unable to pinch the skin can inject directly into the thigh without pinching if needed.Hold the skin pinched until Rasuvo is removed from the skin after the injection.Position the uncapped transparent end of the Rasuvo auto-injector perpendicular (at a 90 degree angle) to the skin (see Figure G).Without pressing the button, push Rasuvo firmly onto your skin until you feel the stop point in order to unlock the yellow injection button (see Figure G).If you are unable to push Rasuvo to the stop-point, ask a caretaker for assistance.STEP 3: Inject Rasuvo
While still holding Rasuvo firmly against the skin, press the yellow injection button with your thumb (see Figure H).You will hear a click which indicates the start of the injection. Hold Rasuvo against the skin until all of the medicine is injected. This can take up to 5 seconds (slowly count 1, 2, 3, 4, 5). To avoid an incomplete injection, do not remove the Rasuvo from the skin before the end of the injection.Look at the transparent control zone while you are injecting to make sure that the entire dose is injected. When the movement stops, the injection is completed.If you have problems with your hearing, slowly count to 5 seconds from the moment you have pressed the button.It is not necessary to keep the button of the Rasuvo pressed down with your thumb after the injection has begun.STEP 4: Check the Transparent Control Zone
Check visually to make sure that there is no liquid left in the syringe inside the transparent control zone.
If there is liquid left, not all of the medicine has been injected correctly. Consult your doctor or health care professional immediately. Do not use another Rasuvo, unless advised by your doctor.STEP 5: Dispose of Rasuvo
Each Rasuvo can be used only 1 time.Do not throw away in the household trash. Put used Rasuvo in an FDA-cleared sharps disposal container right away after use.If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:made of a heavy-duty plasticcan be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come outupright and stable during useleak-resistantproperly labeled to warn of hazardous waste inside the containerWhen your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:How should I store Rasuvo?
Store Rasuvo at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze.Keep Rasuvo out of the light.Always keep your Rasuvo out of the reach of children.This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Medac Pharma Inc.
29 N Wacker Drive, Suite 704
Chicago, IL 60606
11/2014
PRINCIPAL DISPLAY PANEL - 7.5 mg Auto-Injector Carton
NDC 59137-505-04
Rx only
7.5 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
7.5 mg per 0.15 mL
This carton contains 4 pre-assembled 0.15 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-510-04
Rx only
10 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
10 mg per 0.20 mL
This carton contains 4 pre-assembled 0.20 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-515-04
Rx only
12.5 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
12.5 mg per 0.25 mL
This carton contains 4 pre-assembled 0.25 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-520-04
Rx only
15 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
15 mg per 0.30 mL
This carton contains 4 pre-assembled 0.30 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-525-04
Rx only
17.5 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
17.5 mg per 0.35 mL
This carton contains 4 pre-assembled 0.35 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.
NDC 59137-530-04
Rx only
20 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
20 mg per 0.40 mL
This carton contains 4 pre-assembled 0.40 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-535-04
Rx only
22.5 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
22.5 mg per 0.45 mL
This carton contains 4 pre-assembled 0.45 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-540-04
Rx only
25 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
25 mg per 0.50 mL
This carton contains 4 pre-assembled 0.50 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.

NDC 59137-550-04
Rx only
30 mg
Rasuvo®
methotrexate
injection
Rasuvo® (methotrexate) injection
30 mg per 0.60 mL
This carton contains 4 pre-assembled 0.60 mL pre-filled Rasuvo auto-injectors.
FOR SUBCUTANEOUS USE ONLY
See full prescribing information for dosage and administration.
Read enclosed patient package insert carefully before using.
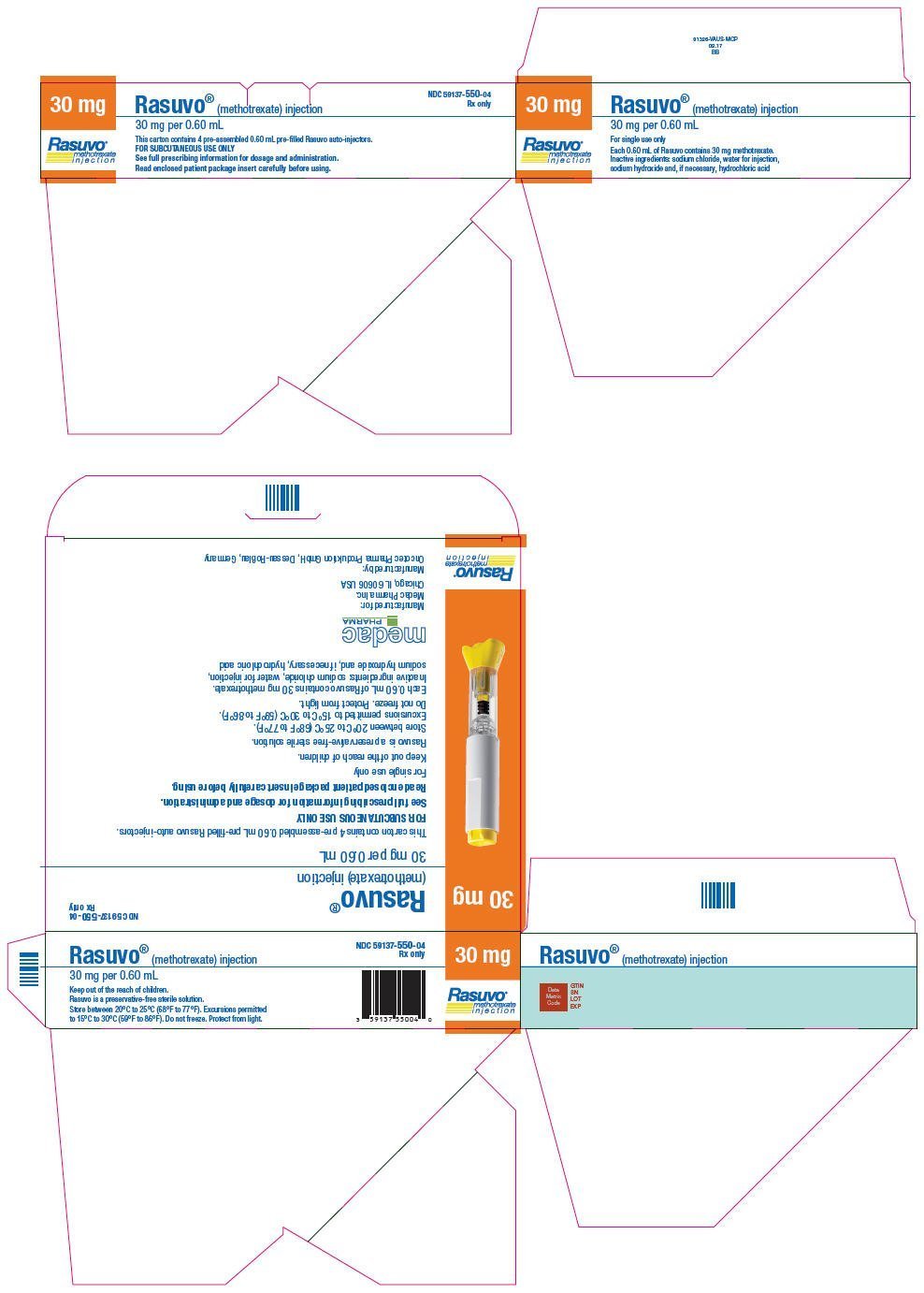
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
RASUVO methotrexate injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - Medac Pharma, Inc (078811131) |