

DUPIXENT 度普利尤单抗注射液




| 通用中文 | 度普利尤单抗注射液 | 通用外文 | Dupilumab |
| 品牌中文 | 达必妥 | 品牌外文 | DUPIXENT |
| 其他名称 | |||
| 公司 | 赛诺菲/再生元(SANOFI) | 产地 | 法国(France) |
| 含量 | 150mg/ml | 包装 | 300mg/2ml/2支/盒 |
| 剂型给药 | 储存 | 2度-8度(冰箱冷藏,禁止冷冻) | |
| 适用范围 | 严重的慢性皮肤炎症,成年人患有慢性鼻炎、鼻窦炎合并鼻息肉。 | ||
| 通用中文 | 度普利尤单抗注射液 |
| 通用外文 | Dupilumab |
| 品牌中文 | 达必妥 |
| 品牌外文 | DUPIXENT |
| 其他名称 | |
| 公司 | 赛诺菲/再生元(SANOFI) |
| 产地 | 法国(France) |
| 含量 | 150mg/ml |
| 包装 | 300mg/2ml/2支/盒 |
| 剂型给药 | |
| 储存 | 2度-8度(冰箱冷藏,禁止冷冻) |
| 适用范围 | 严重的慢性皮肤炎症,成年人患有慢性鼻炎、鼻窦炎合并鼻息肉。 |
![]() 核准日期:2020 年 6 月 17 日 修改日期:
核准日期:2020 年 6 月 17 日 修改日期:
请仔细阅读说明书并在医师指导下使用。
【药品名称】
通用名称:度普利尤单抗注射液 商品名称:达必妥®/ Dupixent® 英文名称:Dupilumab Injection 汉语拼音:Dupuliyou Dankang Zhusheye
【成份】
本品活性成份为度普利尤单抗。度普利尤单抗系采用中国仓鼠卵巢细胞
(CHO-K1)表达制备的靶向白细胞介素-4 受体亚基α(IL-4R-α)的全人单克 隆抗体(IgG4 型),能够抑制 IL-4/IL-13 信号传导。
辅料:组氨酸、盐酸组氨酸、盐酸精氨酸、聚山梨酯 80、醋酸钠、冰醋酸、 蔗糖和注射用水。
【性状】
透明至略带乳白色、无色至淡黄色溶液,无可见颗粒。
【适应症】
本品用于治疗外用处方药控制不佳或不建议使用外用处方药的成人中重度 特应性皮炎。本品可与或不与外用皮质类固醇联合使用。
【规格】
300 mg (2.0 mL)/支(预充式注射器)
【用法用量】
本品应由具有适应症诊断和治疗经验的医疗卫生专业人员处方。 剂量
推荐成人患者使用本品的初始剂量为 600mg (300mg 注射两次),继以每两周
一次给予 300mg,皮下注射给药。
本品可与或不与外用皮质类固醇联合使用。可使用外用钙调神经磷酸酶抑制 剂,但应仅限于问题部位,如面部、颈部、褶皱区域和生殖器部位。
本品治疗特应性皮炎 16 周后无效的患者应考虑停止治疗。一些在初始治疗
中部分应答的患者可能会在 16 周后的继续治疗中获得病情改善。若本品治疗必 须中止,患者仍能成功接受重新治疗。
给药方法
皮下使用。本品通过皮下注射至大腿或腹部,肚脐周围 5 厘米以内的区域除 外。如果由他人进行注射,也可注射于上臂。
对于 600mg 初始剂量,应在不同的注射部位接连注射两次 300mg 本品。 建议每次注射时轮换注射部位。本品不应注射至脆弱、受损或有瘀伤、疤痕
的皮肤上。
在医疗卫生专业人员认可的情况下,患者可以自行注射本品,或者由患者的 看护人员进行给药。在使用前,应根据包装说明书中的使用说明(见文末图示), 对患者和/或看护人员,提供适当的准备和使用本品的培训。
错过用药
如果错过一次用药,应尽快给药。此后,恢复在规定时间内的定期给药。
特殊人群
老年患者(≥65岁)
对于老年患者,不建议调整剂量(见【药代动力学】)。
轻度或中度肾损害患者不需要调整剂量。本品在严重肾损害患者中的数据极 其有限(见【药代动力学】)。
尚无本品在肝功能损害患者中的数据(见【药代动力学】)。
不建议根据体重调整剂量(见【药代动力学】)。
【不良反应】 安全性特征概要
最常见的不良反应是注射部位反应、结膜炎、睑缘炎和口腔疱疹。特应性皮 炎开发项目中报告的血清病/血清病样反应病例非常罕见(见【注意事项】)。
在单药治疗研究中,因不良事件中止治疗的患者比例:安慰剂组为 1.9%, 度普利尤单抗 300mg 每两周一次 (Q2W) 组为 1.9%,度普利尤单抗 300mg 每 一周一次 (QW) 组为 1.5%。在联用外用皮质类固醇 (TCS) 的研究中,因不良事 件中止治疗的患者比例:安慰剂+ TCS 组为 7.6%,度普利尤单抗 300 mg Q2W +
TCS 组为 1.8%,度普利尤单抗 300 mg QW + TCS 组为 2.9%。
不良反应列表
在四项随机、双盲、安慰剂对照研究(分别为 SOLO1, SOLO2, CHRONOS 和 CAFÉ 研究)和一项剂量范围研究(R668-AD-1021 研究)中,评估本品对中 度至重度特应性皮炎患者的安全性。在这 5 项试验中,1689 例受试者接受本品 皮下注射治疗,联合或不联合外用皮质类固醇 (TCS) 。共有 305 例患者接受本 品治疗至少 1 年。
表 1 中列出了按系统器官分类和频率划分的在特应性皮炎临床试验中观察 到的不良反应,使用以下类别:十分常见(≥1/ 10)、常见(≥1/ 100 至<1/10)、偶见 (≥1/ 1,000 至<1/100)、罕见(≥1/ 10,000 至<1/1,000)、十分罕见(<1/10,000)。在每个 频率分组中,不良反应以严重程度递减的顺序呈现。
表 1 特应性皮炎治疗中的不良反应列表
|
MedDRA 系统器官分类 |
频率 |
不良反应 |
|
感染及侵染类疾病 |
常见 |
结膜炎 口腔疱疹 |
|
血液及淋巴系统疾病 |
常见 |
嗜酸性粒细胞增多症 |
|
免疫系统疾病 |
十分罕见 |
血清病/血清病样反应 |
|
各类神经系统疾病 |
常见 |
头痛 |
|
眼器官疾病 |
常见 |
过敏性结膜炎 眼部瘙痒 睑缘炎 |
|
全身性疾病及给药部位各种 反应 |
十分常见 |
注射部位反应 |
特定不良反应描述
罕见本品给药后的血清病/血清病样反应和速发过敏反应病例报告(见【注 意事项】)。
接受本品治疗的特应性皮炎患者出现结膜炎的频率升高。大部分患者的结膜
炎在治疗期间痊愈或好转。(见【注意事项】)。
在 16 周特应性皮炎的单药治疗研究中,度普利尤单抗组疱疹性湿疹报告比 例<1%,安慰剂组报告比例<1%。在 52 周特应性皮炎的度普利尤单抗 + TCS 研 究中,度普利尤单抗 + TCS 组疱疹性湿疹报告比例为 0.2%,安慰剂+ TCS 组报 告比例为 1.9%。
本品治疗患者和安慰剂治疗患者相比,嗜酸粒细胞计数较基线的平均初始增 长更高。在研究治疗期,嗜酸粒细胞计数下降到接近基线水平。
据报告,度普利尤单抗治疗患者治疗中出现的嗜酸粒细胞增多症(≥5,000 细胞/mcL)的比例<1%,安慰剂治疗患者无报告。
在 16 周特应性皮炎的单药治疗研究中,安慰剂组有 1.0%的患者报告严重感 染,度普利尤单抗组有 0.5%的患者报告严重感染。在 52 周特应性皮炎的 CHRONOS 研究中,安慰剂组有 0.6%的患者报告严重感染,度普利尤单抗组有 0.2%的患者报告严重感染。
与所有治疗性蛋白一致,本品也可能具有免疫原性。
抗药抗体 (ADA) 反应通常不会影响本品的暴露、安全性或有效性。 在为期 52 周的研究中,安慰剂组中约 3%的患者、本品组中 2%的患者的抗
药抗体(ADA)反应持续超过 12 周。在这些患者中,安慰剂组有 0.7%的患者,
本品治疗组有 0.2%的患者还具有中和抗体反应,这些反应通常不伴有疗效丧失。
在总体暴露汇总中,少于 0.1%的患者表现出高滴度的 ADA 反应,伴有暴露 和疗效降低。此外,有 1 例血清病患者和 1 例血清病样反应(<0.1%)患者伴有 高滴度 ADA(见【注意事项】)
【禁忌】
对本品活性成分或者其他任何辅料有超敏反应者禁用。
【注意事项】 超敏反应
如果发生全身性超敏反应 (速发型或迟发型) ,应立即停用本品并开始适当 的治疗。本品给药后,特应性皮炎开发项目中报告的血清病/血清病样反应病例
非常罕见。(见【不良反应】)。
蠕虫感染
已知蠕虫感染的患者被排除参加临床研究。本品通过抑制 IL-4/IL-13 信号传 导,可能会影响针对蠕虫感染的免疫应答。原先存在蠕虫感染的患者应在开始使 用本品前进行治疗。如果患者在接受本品治疗期间感染蠕虫,并且对抗蠕虫治疗 无反应,则应停止本品治疗,直至感染消除。
结膜炎相关事件
接受本品治疗的患者,如果发生结膜炎且经标准治疗不能缓解,则应接受眼 科检查(见【不良反应】)。
合并哮喘的特应性皮炎患者
在未咨询医生的情况下,接受本品治疗中度至重度特应性皮炎且合并哮喘的 患者不应调整或停止哮喘治疗方案。停用本品后,应仔细监测合并哮喘患者。
疫苗接种
本品给药时,不得同时给予活疫苗和减毒活疫苗,因为尚未确定此类操作的 临床安全性和疗效。已评估了对 TdaP 疫苗 (破伤风、白喉和百日咳三联疫苗) 和 脑膜炎球菌多糖疫苗的免疫应答(见【药物相互作用】)。建议在本品治疗前,患 者应已根据当前免疫指南完成活疫苗和减毒活疫苗接种。
钠含量
本品每 300mg 剂量含钠低于 1mmol (23mg),即基本“不含钠”。
对驾驶和操作机器能力的影响
本品对驾驶或操作机器能力无影响或可以忽略不计。
配伍禁忌
在无配伍禁忌研究的情况下,本品不得与其他药品混和。
【孕妇及哺乳期妇女用药】 妊娠
本品在孕妇使用的数据非常有限。就生殖毒性而言,动物研究并未提示直接 或间接的有害影响(见【药理毒理】)。本品是重组人 IgG4 单克隆抗体,已知人 IgG 抗体可穿过胎盘屏障,因此本品可能从母体传输至发育中的胎儿。只有证明 潜在获益大于胎儿潜在风险时,才可在妊娠期间使用本品。
哺乳
尚不清楚本品是否在人乳中排泄或摄入后全身吸收,但已知母体 IgG 存在 于母乳中。做出是否停止母乳喂养或停止本品治疗的决定必须考虑母乳喂养对儿 童的益处以及治疗对母亲的益处。
生育力
动物研究未显示生育力受损(见【药理毒理】)。
【儿童用药】
本品在中国 18 岁以下特应性皮炎儿童患者的安全性和有效性尚未确立。在
国外本品已完成用于治疗 12-17 岁特应性皮炎儿童患者的临床试验,包括患者药 代动力学(PK)研究和多中心随机对照的有效性/安全性研究。
【老年用药】
群体药代动力学分析未发现年龄对本品全身暴露有任何具有临床意义的影 响。见【药代动力学】
【药物相互作用】
在使用本品每周治疗一次,共 16 周的方案治疗特应性皮炎患者的研究中, 评估了疫苗的免疫应答。经 12 周本品给药后,患者接种 Tdap 疫苗 (T 细胞依赖 性) 和脑膜炎球菌多糖疫苗 (T 细胞非依赖性) ,并在 4 周后评估免疫应答。本 品治疗组和安慰剂组患者对破伤风疫苗和脑膜炎球菌多糖疫苗的抗体反应相似。 在研究中未观察到非活疫苗和本品之间的不良相互作用。
因此,接受本品治疗的患者可同时接种灭活或非活疫苗。关于活疫苗的信息, 见【注意事项】。
在一项特应性皮炎患者的临床研究中,评估了本品对 CYP 底物药代动力学 (PK)的影响。该研究收集的数据并未显示本品对 CYP1A2、CYP3A、CYP2C19、 CYP2D6 或 CYP2C9 活性具有临床相关的影响。
【药物过量】
本品用药过量无特殊治疗方法。如果出现药物过量,应监测患者是否有任何 不良反应体征或症状,并立即采取适当的对症治疗。
【临床试验】
在 全球 三项 关 键性随 机 、双盲 、 安慰剂 对 照研究 (SOLO1 , SOLO2 和 CHRONOS)中评估了本品单药治疗以及联合外用皮质类固醇的疗效和安全性, 共有 2119 例年龄在 18 岁及以上的中度至重度特应性皮炎(AD)患者参与,中至 重度特应性皮炎定义为研究者总体评估(IGA)评分≥3,湿疹面积和严重程度指数
(EASI)评分≥16,最低受累及体表面积(BSA)≥10%。入选至这三项研究符合条件 的患者既往使用外用药物疗效不足。
在所有三项研究中,患者 1) 在第 1 天接受初始剂量为 600mg 的度普利尤单 抗 (300mg,两次注射) ,然后每两周一次 300mg (Q2W) ;2) 在第 1 天接受初 始剂量为 600mg 的度普利尤单抗,随后每周一次 300mg (QW) ;或 3) 接受相匹 配的安慰剂。在所有研究中,本品通过皮下 (SC) 注射给药。如果需要控制无法 耐受的特应性皮炎症状,则允许患者根据研究者的判断接受补救治疗 (包括更高 效的外用皮质类固醇或全身性免疫抑制剂)。接受补救治疗的患者视为非应答者。
SOLO1 研究入选了 671 例患者 (224 例接受安慰剂治疗,224 例接受度普利 尤单抗 300mg Q2W 治疗,223 例接受度普利尤单抗 300mg QW 治疗) ,治疗周 期为 16 周。
SOLO2 研究入选了 708 例患者 (236 例接受安慰剂治疗,233 例接受度普利 尤单抗 300mg Q2W 治疗,239 例接受度普利尤单抗 300mg QW 治疗),治疗周 期为 16 周。
CHRONOS 研究入选了 740 例患者(315 例接受安慰剂+ TCS 治疗,106 例接 受度普利尤单抗 300mg Q2W + TCS 治疗,319 例接受度普利尤单抗 300mg QW
+ TCS 治疗),治疗周期为 52 周。接受本品或安慰剂联合使用 TCS 的患者从基线 开始使用标准化方案。还允许患者使用外用钙调神经磷酸酶抑制剂(TCI)。
终点
在所有三项关键性研究中,并列主要终点是自基线至第 16 周时 IGA 0 或 1(“清除”或“几乎清除”)且 0-4 IGA 评分至少下降 2 分的患者比例、EASI 至少改 善 75%(EASI-75)的患者比例。其他评估结果包括自基线至第 16 周 EASI 至少改 善 50%和 90%(分别为 EASI-50 和 EASI-90)的患者比例、由峰值瘙痒数字评估量 表(NRS)测量的瘙痒减少程度以及特应性皮炎评分(SCORAD)变化百分比。其他 次要终点包括患者湿疹自我评价(POEM)、皮肤病生活质量指数(DLQI)和医院焦 虑抑郁量表(HADS)评分自基线至第 16 周的平均变化。在 CHRONOS 研究中, 还在第 52 周时评估疗效。
基线特征
在单药治疗研究中(SOLO1 和 SOLO2),所有治疗组的平均年龄为 38.3 岁, 平均体重为 76.9kg,女性占 42.1%,白种人占 68.1%,亚洲人占 21.8%,黑人占 6.8%。在这些研究中,51.6%的患者基线 IGA 评分为 3 分(中度 AD),48.3%的患 者基线 IGA 评分为 4(重度 AD),32.4%的患者既往接受全身性免疫抑制剂治疗。 基线平均 EASI 评分为 33.0,基线瘙痒 NRS 周平均值为 7.4,基线平均 SCORAD 评分为 67.8,基线平均 POEM 评分为 20.5,基线平均 DLQI 为 15.0,基线平均 HADS 总评分为 13.3。
在联合 TCS 的研究(CHRONOS)中,所有治疗组的平均年龄为 37.1 岁,平均 体重为 74.5kg,女性占 39.7%,白种人占 66.2%,亚洲人占 27.2%,黑人占 4.6%。 在这项研究中,53.1%的患者基线 IGA 评分为 3 分,46.9%的患者基线 IGA 评分 为 4 分,33.6%的患者既往接受全身性免疫抑制剂治疗。基线平均 EASI 评分为 32.5,基线周瘙痒 NRS 为 7.3,基线平均 SCORAD 评分为 66.4,基线平均 POEM 评分为 20.1,基线平均 DLQI 为 14.5,基线平均 HADS 总评分为 12.7。
临床应答
16 周单药治疗研究(SOLO1 和 SOLO2)
在 SOLO1 和 SOLO2 研究中,从基线至第 16 周,与安慰剂组相比,随机分 配至本品治疗的患者显著有更大比例患者实现 IGA 0 或 1 应答、EASI-75 和/或 瘙痒 NRS 改善≥ 4 分(见表 2)。
与安慰剂组相比,随机分配至本品治疗的患者显著有更大比例患者实现瘙痒 NRS 迅速改善(定义为早在第 2 周改善≥4 分;p <0.01),在整个治疗期,瘙痒 NRS 应答患者比例继续增加。瘙痒 NRS 改善伴随特应性皮炎客观体征的改善。
图 1 和图 2 分别显示了自基线至第 16 周时的 EASI 平均变化百分比和 NRS
平均变化百分比。
表 2 第 16 周本品单药治疗的疗效结果(FAS)
|
|
SOLO1 (FAS)a |
SOLO2 (FAS)a |
||||
|
|
安慰剂 |
度普利尤 单抗 300 mg Q2W |
度普利尤 单抗 300 mg QW |
安慰剂 |
度普利尤 单抗 300 mg Q2W |
度普利尤 单抗 300 mg QW |
|
随机分组的患者 数量 |
224 |
224 |
223 |
236 |
233 |
239 |
|
IGA 0 或 1b,应 答者百分比 c |
10.3% |
37.9%e |
37.2%e |
8.5% |
36.1%e |
36.4%e |
|
EASI-50,应答 者百分比 c |
24.6% |
68.8%e |
61.0%e |
22.0% |
65.2%e |
61.1%e |
|
EASI-75,应答 者百分比 c |
14.7% |
51.3%e |
52.5%e |
11.9% |
44.2%e |
48.1%e |
|
EASI-90,应答 者百分比 c |
7.6% |
35.7%e |
33.2%e |
7.2% |
30.0%e |
30.5%e |
|
EASI,自基线的 |
-37.6% |
-72.3%e |
-72.0%e |
-30.9% |
-67.1%e |
-69.1%e |
|
LS 平均变化百 |
(3.28) |
(2.63) |
(2.56) |
(2.97) |
(2.52) |
(2.49) |
|
分比(+/-SE) |
||||||
|
SCORAD,自基 |
-29.0% |
-57.7%e |
-57.0%e |
-19.7% |
-51.1%e |
-53.5%e |
|
线的 LS 平均变 |
(3.21) |
(2.11) |
(2.11) |
(2.52) |
(2.02) |
(2.03) |
|
化百分比(+/-SE) |
||||||
|
瘙痒 NRS,自基 |
-26.1% |
-51.0%e |
-48.9%e |
-15.4% |
-44.3%e |
-48.3%e |
|
线的 LS 平均变 化百分比(+/-SE) |
(3.02) |
(2.50) |
(2.60) |
(2.98) |
(2.28) |
(2.35) |
|
基线瘙痒 NRS 评分≥4 的患者数 量 |
212 |
213 |
201 |
221 |
225 |
228 |
|
瘙痒 NRS (改 善>4 分),应答 者百分比 c,d |
12.3% |
40.8%e |
40.3%e |
9.5% |
36.0%e |
39.0%e |
LS =最小二乘法;SE =标准误
a 全分析集(FAS)包括所有随机分组的患者。
b 应答者定义为 IGA 0 或 1(“清除”或“几乎清除”)且 0-4 IGA 评分降低≥ 2 分的患者。
c 接受过补救治疗或数据缺失的患者视为非应答者。
d 第 2 周时,相比于安慰剂组,度普利尤单抗组中显著有更多患者瘙痒 NRS 评分改善≥4 分(P
<0.01)。
e p 值<0.0001。
图 1 SOLO1a 和 SOLO2a(FAS)b 研究中 EASI 自基线的平均变化百分比
LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 全分析集(FAS)包括所有随机分组的患者。
图 2 SOLO1a 和 SOLO2a(FAS)b 研究中 NRS 自基线的平均变化百分比

LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 全分析集(FAS)包括所有随机分组的患者。
SOLO1 和 SOLO2 亚组(体重、年龄、性别、种族和背景治疗,包括免疫抑 制剂)的治疗效果与总体研究人群结果一致。
52 周联合 TCS 的研究(CHRONOS)
从基线至第 16 周及第 52 周,与安慰剂+TCS 组相比,随机分配至度普利尤 单抗 300 mg Q2W + TCS 治疗的患者显著有更大比例患者实现 IGA 0 或 1 应答, EASI-75 和/或瘙痒 NRS 改善≥ 4 分(见表 3)。
与安慰剂+TCS 组相比,随机分配至度普利尤单抗+TCS 治疗的患者显著有 更大比例患者实现瘙痒 NRS 迅速改善(定义为早在第 2 周改善≥4 分; p <0.05),在 整个治疗期,瘙痒 NRS 应答患者的比例继续增加。瘙痒 NRS 改善伴随特应性皮 炎客观体征的改善。
图 3 和图 4 分别显示了在 CHRONOS 研究中直至第 52 周时的 EASI 较基线 平均变化百分比和 NRS 较基线平均变化百分比。
表 3 CHRONOS 研究中本品联合使用 TCSa 第 16 周和第 52 周的疗效结果
 第 16 周(FAS)b 第 52 周(第 52 周的 FAS)b
第 16 周(FAS)b 第 52 周(第 52 周的 FAS)b
|
|
安慰 剂 + TCS |
度普利尤 单抗 300 mg Q2W + TCS |
度普利尤 单抗 300 mg QW + TCS |
安慰 剂 + TCS |
度普利尤 单抗 300 mg Q2W + TCS |
度普利尤 单抗 300 mg QW + TCS |
|
随机分组的患者数量 |
315 |
106 |
319 |
264 |
89 |
270 |
|
IGA 0 或 1c,应答者百 |
12.4% |
38.7%f |
39.2%f |
12.5% |
36.0%f |
40.0%f |
|
分比 d |
|
|
|
|
|
|
|
EASI-50,应答者百分 |
37.5% |
80.2%f |
78.1%f |
29.9% |
78.7%f |
70.0%f |
|
比 d |
|
|
|
|
|
|
|
EASI-75,应答者百分 |
23.2% |
68.9%f |
63.9%f |
21.6% |
65.2%f |
64.1%f |
|
比 d |
|
|
|
|
|
|
|
EASI-90,应答者百分 |
11.1% |
39.6%f |
43.3%f |
15.5% |
50.6%f |
50.7%f |
|
比 d |
|
|
|
|
|
|
|
EASI,自基线的 LS |
- |
-80.5%f |
-81.5%f |
- |
-84.9%g |
-87.8%h |
|
平均变化百分比(+/- |
48.4% |
(6.34) |
(5.78) |
60.9% |
(6.73) |
(6.19) |
|
SE) |
(3.82) |
|
|
(4.29) |
|
|
|
SCORAD,自基线的 |
- |
-63.9%f |
-65.9%f |
- |
-69.7%f |
-70.4%f |
|
LS 平均变化百分比 |
36.2% |
(2.52) |
(1.49) |
47.3% |
(3.06) |
(1.72) |
|
(+/-SE) |
(1.66) |
|
|
(2.18) |
|
|
|
瘙痒 NRS,自基线的 |
- |
-56.6%f |
-57.1%f |
- |
-57.0%i |
-56.5%f |
|
LS 平均变化百分比 |
30.3% |
(3.95) |
(2.11) |
31.7% |
(6.17) |
(3.26) |
|
(+/-SE) |
(2.36) |
|
|
(3.95) |
|
|
|
基线瘙痒 NRS 评分≥4 的患者数量 |
299 |
102 |
295 |
249 |
86 |
249 |
|
瘙痒 NRS(改善>4 |
19.7% |
58.8%f |
50.8%f |
12.9% |
51.2%f |
39.0%f |
|
分),应答者百分比 d, e |
|
|
|
|
|
|
LS =最小二乘法;SE =标准误
a 所有患者均接受外用皮质类固醇背景治疗,并允许患者使用外用钙调神经磷酸酶抑制剂。 b 全分析集(FAS)包括所有随机分组的患者。第 52 周的 FAS 包括所有在主要分析截止日期之 前至少一年随机分组的患者。
c 应答者定义为 IGA 0 或 1(“清除”或“几乎清除”)且 0-4 IGA 评分降低≥ 2 分的患者。
d 接受过补救治疗或数据缺失的患者视为非应答者。
e 第 2 周时,相比于安慰剂组,度普利尤单抗组中显著有更多患者瘙痒 NRS 评分改善≥4 分
(P <0.05)。
f p 值<0.0001 g p 值= 0.0015 h p 值= 0.0003 i p 值= 0.0005
图 3 CHRONOSa 研究中(第 52 周的 FAS)bEASI 自基线的平均变化百分比

LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 第 52 周的 FAS 包括所有在主要分析截止日期之前至少一年随机分组的患者。
图 4 CHRONOSa 研究中(第 52 周的 FAS)b NRS 自基线的平均变化百分比
CHRONOS

LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 第 52 周的 FAS 包括所有在主要分析截止日期之前至少一年随机分组的患者。
CHRONOS 亚组(体重、年龄、性别、种族和背景治疗,包括免疫抑制剂)的 治疗效果与总体研究人群结果一致。
(CAFE 研究)
CAFE 研究评估了成年 AD 患者使用本品相比安慰剂(联合使用 TCS)治疗 16 周期间的疗效,这些患者口服环孢素不能充分控制病情、或不耐受环孢素或当前 使用环孢素为禁忌或医学上不适用。
共有 325 例患者入选,其中 210 例患者既往暴露于环孢素,115 例患者因在 医学上不适用环孢素治疗从未暴露于环孢素。平均年龄为 38.4 岁,女性占 38.8%, 基线平均 EASI 评分为 33.1,平均 BSA 为 55.7,基线瘙痒 NRS 周平均值为 6.4, 基线平均 SCORAD 评分为 67.2,基线平均 DLQI 为 13.8 。
主要终点是第 16 周时实现 EASI-75 的患者比例。 表 4 总结了 16 周 CAFE 研究的主要和次要终点。
表 4 CAFE 研究中主要和次要终点结果
|
|
安慰剂 + TCS |
度普利尤单抗 300 mg Q2W + TCS |
度普利尤单抗 300 mg QW+TCS |
|
随机分组的患者数量 |
108 |
107 |
110 |
|
EASI-75, 应答者百分比 |
29.6% |
62.6% |
59.1% |
|
EASI, 自基线的 LS 平均变化 |
-46.6 |
-79.8 |
-78.2 |
|
百分比(+/-SE) |
(2.76) |
(2.59) |
(2.55) |
|
瘙痒 NRS,自基线的平均变化 |
-25.4% |
-53.9% |
-51.7% |
|
百分比(+/-SE) |
(3.39) |
(3.14) |
(3.09) |
|
SCORAD, 自基线的 LS 平均 |
-29.5% |
-62.4% |
-58.3% |
|
变化百分比(+/-SE) |
(2.55) |
(2.48) |
(2.45) |
|
DLQI, 自基线的 LS 平均变化 |
-4.5 |
-9.5 |
-8.8 |
|
百分比(+/-SE) |
(0.49) |
(0.46) |
(0.45) |
(p 值均<0.0001)
在 52 周 CHRONOS 研究中,类似于 CAFE 研究人群的患者亚组,接受度普 利尤单抗 300mg Q2W 治疗的患者有 69.6%在第 16 周时达到 EASI-75,而接受 安慰剂治疗的患者有 18.0%达到 EASI-75;第 52 周时,接受度普利尤单抗 300mg Q2W 治疗的患者有 52.4%达到 EASI-75,而接受安慰剂治疗的患者有 18.6%达到 EASI-75。在该亚集中,度普利尤单抗 300mg Q2W 治疗组和安慰剂治疗组瘙痒 NRS 自基线变化的百分比在第 16 周分别为-51.4% 和 -30.2%,在第 52 周分别 为-54.8%和 -30.9%。
疗效的维持和持久性(SOLO CONTINUE 研究)
为了评估疗效的维持和持久性,在 SOLO1 和 SOLO2 研究中接受本品治疗 16 周并实现 IGA 0 或 1 或 EASI-75 的受试者在 SOLO CONTINUE 研究中再随机 分配,再进行额外的 36 周本品或安慰剂治疗,以达到为期 52 周的累积研究治
疗。在第 51 或 52 周评估终点。
并列主要终点是 EASI 评分相对 SOLO1 和 SOLO2 研究基线的百分比变化
在基线(第 0 周)和第 36 周之间的差异,以及基线时 EASI-75 患者中第 36 周时仍 达 EASI-75 患者的百分比。
继续接受 SOLO1 和 SOLO2 研究中相同剂量方案治疗的患者(300 mg Q2W 或 300 mg QW)显示维持临床反应的最佳效果,而其他剂量方案的疗效以剂量依 赖性方式下降。
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
†P<0.05, *P<0.01, **P<0.001, ***P≤0.0001
在 SOLO CONTINUE 研究中,观察到治疗中出现的 ADA 阳性率随着给药 间隔增加而升高的趋势。治疗中出现的 ADA:QW:1.2%、Q2W:4.3%、Q4W: 6.0%、Q8W:11.7%。持续超过 12 周的 ADA 反应:QW:0.0%、Q2W:1.4%、 Q4W:0.0%、Q8W:2.6%。
在单药治疗研究(SOLO1 和 SOLO2)中,第 16 周时,与安慰剂组比较,度普 利尤单抗 300 mg Q2W 和 300 mg QW 组分别显著改善了患者报告的症状以及 AD 对睡眠和健康相关生活质量的影响(由 POEM 和 DLQI 总评分衡量)。与安慰 剂组相比,从基线至第 16 周,本品给药组中有更多比例患者 POEM 和 DLQI 总 分出现有临床意义的降低(每种评分均定义为改善≥4 分)。此外,与安慰剂组相比, 第 16 周时,度普利尤单抗组由 HADS 总评分衡量的焦虑和抑郁症状显著减少。 在基线 HADS 焦虑或 HADS 抑郁子量表评分≥8 分(焦虑或抑郁的界值)的患者子
集中,与安慰剂组相比,第 16 周时度普利尤单抗组中有更多比例的患者达到
HADS 焦虑和 HADS 抑郁评分<8(见表 6)。
表 6 第 16 周时度普利尤单抗单药治疗的其他次要终点结果
|
|
单药治疗 |
|||||
|
|
SOLO1 第 16 周时 |
SOLO2 第 16 周时 |
||||
|
|
安慰 |
度普利尤单抗 |
度普利尤单 |
安慰 |
度普利尤单抗 |
度普利尤单 |
|
剂 |
300 mg Q2W |
抗 |
剂 |
300 mg Q2W |
抗 |
|
|
300 mg QW |
300 mg QW |
|||||
|
随机分组的患者数量 |
224 |
224 |
223 |
236 |
233 |
239 |
|
DLQI,自基线的 LS 平 |
-5.3 |
-9.3a |
-9.0a |
-3.6 |
-9.3a |
-9.5a |
|
均变化 (SE) |
(0.50) |
(0.40) |
(0.40) |
(0.50) |
(0.38) |
(0.39) |
|
POEM, 自基线的 LS |
-5.1 |
-11.6a |
-11.0a |
-3.3 |
-10.2a |
-11.3a |
|
平均变化 (SE) |
(0.67) |
(0.49) |
(0.50) |
(0.55) |
(0.49) |
(0.52) |
|
HADS,自基线的 LS |
-3.0 |
-5.2b |
-5.2b |
-0.8 |
-5.1a |
-5.8a |
|
平均变化, (SE) |
(0.65) |
(0.54) |
(0.51) |
(0.44) |
(0.39) |
(0.38) |
|
|
||||||
|
基线时 DLQI ≥4 的患 者数量 |
213 |
209 |
209 |
225 |
223 |
234 |
|
DLQI(改善≥4 分), 应 答者百分比 |
30.5% |
64.1%a |
58.4%a |
27.6% |
73.1%a |
62.0%a |
|
|
||||||
|
基线时 POEM ≥4 的 患者数量 |
223 |
222 |
222 |
234 |
233 |
239 |
|
POEM(改善≥4 分), 应 答者百分比 |
26.9% |
67.6%a |
63.1%a |
24.4% |
71.7%a |
64.0%a |
|
|
||||||
|
基线时 HADS 焦虑评 分≥8 或 HADS 抑郁评 分 ≥8 的患者数量 |
97 |
100 |
102 |
115 |
129 |
136 |
|
达到 HADS 焦虑和 HADS 抑郁评分<8 的 患者,% |
12.4% |
41.0%a |
36.3%b |
6.1% |
39.5%a |
41.2%a |
LS = 最小二乘法; SE =标准误
a p 值<0.0001 b p 值<0.001
在联合 TCS 的研究(CHRONOS)中,第 52 周时,与安慰剂+TCS 组比较,度 普利尤单抗 300 mg Q2W+TCS 和度普利尤单抗 300 mg QW+TCS 组分别显著改 善了患者报告的症状以及 AD 对睡眠和健康相关生活质量的影响(由 POEM 和 DLQI 总评分衡量)。与安慰剂组相比,从基线至第 52 周,度普利尤单抗 300 mg Q2W+TCS 和 300 mg QW+TCS 给药组中,有更多比例患者 POEM 和 DLQI 总分 出现有临床意义的降低(每种评分均定义为改善≥4 分)。此外,与安慰剂+TCS 组 相比,第 52 周时,度普利尤单抗 300 mg Q2W+TCS 和 300 mg QW+TCS 组由
HADS 总评分衡量的焦虑和抑郁症状显著减少。在基线时具有 HADS 焦虑或 HADS 抑郁子量表评分≥8 分(焦虑或抑郁的界值)的患者子集的事后分析中,与安 慰剂+ TCS 组相比,第 52 周时度普利尤单抗 300 mg Q2W + TCS 和 300mg QW
+ TCS 组中有更多比例患者达到 HADS-焦虑和 HADS-抑郁评分<8(参见表 7)。
表 7 CHRONOS 研究中本品伴随 TCS 治疗第 16 周和第 52 周时的其他次要终点结果
|
|
伴随使用 TCS |
|||||
|
|
CHRONOS 第 16 周时 |
CHRONOS 第 52 周时 |
||||
|
|
安慰剂 |
度普利尤单 |
度普利尤单 |
安慰剂 |
度普利尤单 |
度普利尤 |
|
抗 300 mg |
抗 300 mg |
+TCS |
抗 300 mg |
单抗 300 |
||
|
Q2W +TCS |
QW +TCS |
Q2W+ TCS |
mg QW+ |
|||
|
TCS |
||||||
|
随机分组的患者数量 |
315 |
106 |
319 |
264 |
89 |
270 |
|
DLQI, 自基线的 LS |
-5.8 |
-10.0a |
-10.7a |
-7.2 |
-11.4a |
-11.1a |
|
平均变化 (SE) |
(0.34) |
(0.50) |
(0.31) |
(0.40) |
(0.57) |
(0.36) |
|
POEM, 自基线的 LS |
-5.3 |
-12.7a |
-12.9a |
-7.0 |
-14.2a |
-13.2a |
|
平均变化 (SE) |
(0.41) |
(0.64) |
(0.37) |
(0.57) |
(0.78) |
(0.45) |
|
HADS, 自基线的 LS |
-4.0 |
-4.9 |
-5.4c |
-3.8 |
-5.5c |
-5.9b |
|
平均变化 (SE) |
(0.37) |
(0.58) |
(0.35) |
(0.47) |
(0.71) |
(0.42) |
|
|
||||||
|
基线时 DLQI ≥4 的患 者数量 |
300 |
100 |
311 |
254 |
85 |
264 |
|
DLQI (改善≥4 分), 应 答者百分比 |
43.0% |
81.0 %a |
74.3 %a |
30.3% |
80.0 %a |
63.3 %a |
|
|
||||||
|
基线时 POEM ≥4 的患 者数量 |
312 |
106 |
318 |
261 |
89 |
269 |
|
POEM (改善≥4 分), 应 答者百分比 |
36.9% |
77.4 %a |
77.4 %a |
26.1% |
76.4 %a |
64.7 %a |
|
|
||||||
|
基线时 HADS 焦虑 评分≥8 或 HADS 抑郁 评分 ≥8 的患者数量 |
148 |
59 |
154 |
133 |
53 |
138 |
|
达到 HADS 焦虑和 HADS 抑郁评分<8 的 患者百分比 |
26.4% |
47.5%c |
47.4%b |
18.0% |
43.4%b |
44.9%a |
LS =最小二乘法; SE =标准误
a p 值<0.0001 b p 值<0.001 c p 值<0.05
【药理毒理】
药理作用
作用机制
度普利尤单抗是一种全人单克隆抗体(IgG4 型),可通过与白介素-4(IL- 4)和白介素-13(IL-13)受体复合物共享的 IL-4Rα 亚单位特异性结合而抑制 IL-
4 和 IL-13 的信号传导。度普利尤单抗通过 I 型受体抑制 IL-4 信号传导,并通过
II 型受体抑制 IL-4 和 IL-13 信号传导。 炎症是哮喘和特应性皮炎发病机理的重要组成部分。炎症涉及可表达 IL-
4Rα 的多种细胞类型(如:肥大细胞、嗜酸粒细胞、巨噬细胞、淋巴细胞、上皮 细胞、杯状细胞)和炎性介质(如:组胺、类花生酸、白三烯、细胞因子、趋化 因子)。利用度普利尤单抗阻断 IL-4Rα,可抑制 IL-4 和 IL-13 细胞因子诱导的炎 性反应,包括:促炎细胞因子、趋化因子、一氧化氮和 IgE 的释放;但度普利尤 单抗对哮喘的作用机制尚未明确。
毒理研究
遗传毒性 尚未开展动物试验评价度普利尤单抗的致突变性。 生殖毒性
性成熟小鼠皮下注射剂量高达 200 mg/kg/周的抗 IL-4Rα 同源抗体,未见 对生殖器官、月经周期长度或精子分析等生育力指标的影响。
围产期毒性研究中,从器官形成开始至分娩,妊娠食蟹猴皮下注射抗 IL-4Rα 同源抗体 100 mg/kg/周(以 mg/kg 计,为最大推荐人用剂量 MRHD 的 10 倍)。 在出生至 6 月龄胎仔中未见胚胎-胎仔毒性或畸形,或对形态学、功能或免疫学 发育的治疗相关不良影响。
致癌性 尚未开展动物试验评价度普利尤单抗的致癌性。
【药代动力学】 吸收
单次皮下(SC)注射 75-600 mg 剂量的本品后,达到血清峰浓度的中位时间为 3-7 天。通过群体药代动力学(PK)分析确定,皮下注射给药后,估计本品的绝对 生物利用度为 64%。
起始给药剂量 600mg,随后每两周给药 300mg,第 16 周达到稳态浓度。在
所有临床试验中,每两周给予 300 mg 剂量,稳态谷浓度平均值±SD 范围为 73.3
±40.0 mcg/mL 至 79.9±41.4 mcg/mL。
分布
通过群体 PK 分析估计本品的分布容积大约为 4.6L,表明本品主要分布在血 管系统中。
生物转化
未进行特定的代谢研究,因为本品是一种蛋白质。预期本品会降解为小肽和 单个氨基酸。
消除
本品可通过平行线性和非线性途径消除。在较高浓度下,本品主要通过非饱 和的蛋白水解途径进行消除,而在较低浓度时,非线性饱和 IL-4Rα 靶点介导的 消除占优势。在末次稳态剂量给药后,通过群体 PK 分析估计,300mg Q2W 方 案度普利尤单抗浓度降至低于检测下限的中位时间为 10 周,300mg QW 方案为 13 周。
线性/非线性
由于非线性清除,以浓度-时间曲线下面积测量的本品暴露随着单次皮下注 射剂量的增加(75mg-600mg)以高于剂量增加比例的方式增加。
特殊人群
通过群体 PK 分析确定,未发现性别对本品全身暴露有任何临床有意义的影 响。
在 2 期剂量范围研究或 3 期安慰剂对照研究中,共有 1472 例特应性皮炎患
者接受本品治疗,其中共有 67 例患者年龄在 65 岁或以上。虽然老年患者和年轻 特应性皮炎成人患者之间的安全性或有效性没有差异,65 岁及以上的患者人数 不足以确定是否与年轻患者的反应不同。
通过群体 PK 分析确定,未发现年龄对本品全身暴露有任何临床有意义的影 响。然而,本分析中仅有 61 例 65 岁以上的患者。
通过群体 PK 分析确定,未发现种族对本品全身暴露有任何临床有意义的影 响。
作为单克隆抗体,本品预期不会发生显著的肝脏消除。未进行临床研究评估 肝损害对本品药代动力学的影响。
作为单克隆抗体,本品预期不会发生显著的肾脏消除。未进行临床研究评估 肾损害对本品药代动力学的影响。群体 PK 分析未发现轻度或中度肾损害对本品 全身暴露有临床意义的影响。本品在严重肾损害患者中的资料非常有限。
在体重较高的受试者中本品谷浓度较低,对疗效未产生有意义的影响。
在中国健康受试者中单次皮下注射本品 200 mg、300 mg 和 600 mg 后,达到 血清峰浓度的中位时间为 7-8.5 天。血清中功能性度普利尤单抗的平均最大浓度
(Cmax)分别为 25.4、37.2 和 77.3 mg/L,随剂量近似成比例增加。AUC 和 AUClast 以大于剂量比例的方式增加,AUC 的平均值分别为 425,807 和 2150 mg·day/L,AUClast 的平均值分别为 402, 792 和 2110 mg·day/L。
【贮藏】
于 2~8℃避光、密封贮藏,避免冷冻。
运输过程中:冷藏储存(2~8℃),注射器应保存在包装盒内,不能进行冷 冻。
患者使用时:通常情况下需冷藏储存(2~8℃)。如有特殊需要,可在常温
(<25℃)条件下储存 14 天,须避光保存,且不可再返回冷藏储存(2~8℃)。 如果在 14 天内没有使用或储存温度超过 25℃应丢弃。
【包装】
2mL 溶液装于带有一个固定不锈钢针头的预充式注射器(1 型透明玻璃)中, 预充式注射器带有针头防护的安全装置。
包装规格为 2 支/盒。
【有效期】
36 个月。
【执行标准】JS20190073
【批准文号】进口药品注册证号:S20200017
【药品上市许可持有人】
名称: Sanofi-aventis groupe
注册地址:54, rue La Boétie, 75008 Paris, France
【生产企业】
企业名称:Sanofi Winthrop Industrie
生产地址:1051 Boulevard Industriel, 76580 Le Trait, France
【境内联系机构】 名称:赛诺菲(中国)投资有限公司 地址:北京市朝阳区建国路 112 号 7 层 联系方式:800(400)-820-8884
使用本品之前请仔细阅读该说明书的全部内容,因为该说明书中包含重要的 用药信息。
图 1 带有针头防护装置的预充式注射器的示意图

重要信息
该装置为一次性使用的预充式注射器,含有 300mg 用于注射至皮肤下的度 普利尤单抗(皮下注射)。除非您的医疗卫生专业人员对您进行过培训,否则您不 得尝试自行或给他人注射本品。
· 使用注射器之前,请仔细阅读所有使用说明。
· 咨询您的医疗卫生专业人员您需要多长时间注射一次药物。
· 要求您的医疗卫生专业人员在首次注射前向您展示正确使用注射器的方 法。
· 每次注射更换注射部位。
· 如果注射器掉落在坚硬的表面上或损坏,请勿使用注射器。
· 如果针帽缺失或未牢固连接,请勿使用注射器。
· 在准备好注射之前,请勿触碰柱塞杆。
· 请勿透过衣服注射。
· 请勿去除注射器中的气泡。
· 为防止意外的针头损伤,每个预充式注射器都有一个针头防护装置,在 您注射后,针头防护装置会自动启动以覆盖针头。
· 请勿回拉柱塞杆。
· 请勿重复使用注射器。
如何存储度普利尤单抗
· 将注射器置于儿童无法接触的地方。
· 将未使用的注射器存放于原包装盒内,并在 2℃-8℃条件下存放于冰箱 中。
· 请勿将度普利尤单抗于室温(<25℃)下保存超过 14 天。如果您需要从冰 箱中永久取出包装盒,请在外包装盒上的空白处记录取出日期,并在 14 天内使用度普利尤单抗。
· 任何时候请勿振摇注射器。
· 请勿加热注射器。
· 请勿冷冻注射器。
· 请勿将注射器置于阳光直射的地方。
第 1 步:取出注射器 握住注射器主体中部,从包装盒中取出注射器。 ![]() 在准备好注射之前,请勿拔下针帽。
在准备好注射之前,请勿拔下针帽。
![]() 如果注射器掉落到坚硬的表面或损坏,请勿使用。
如果注射器掉落到坚硬的表面或损坏,请勿使用。

第 2 步:准备
确保您有以下几件物品:
· 度普利尤单抗预充式注射器
· 1 块酒精擦拭片*
· 1 个棉球或纱布*
· 防刺穿容器*(请参阅步骤 12)
*不包含在包装盒内的物品
看标签:
· 检查有效期限。
· 检查是否为正确的产品和剂量。
![]() 如果有效期限已过,请勿使用注射器。
如果有效期限已过,请勿使用注射器。 ![]() 请勿将本品于室温下放置超过 14 天。
请勿将本品于室温下放置超过 14 天。

第 3 步:检查
通过注射器的观察窗观察药物:检查液体是否为透明无色至浅黄色。
注意:您可能会看到气泡,此为正常现象。
![]() 如果液体变色或浑浊,或含有块状物或颗粒,请勿使用注射器。
如果液体变色或浑浊,或含有块状物或颗粒,请勿使用注射器。

第 4 步:等待 45 分钟
 将注射器置于平坦表面上至少 45 分钟,使其自然达到室温。
将注射器置于平坦表面上至少 45 分钟,使其自然达到室温。 ![]() 请勿加热注射器。
请勿加热注射器。 ![]() 请勿将注射器置于阳光直射的地方。
请勿将注射器置于阳光直射的地方。 ![]() 请勿将度普利尤单抗于室温下放置超过 14 天。
请勿将度普利尤单抗于室温下放置超过 14 天。
第 5 步:选择 选择注射部位。
· 可以注射至大腿或腹部(肚子),肚脐周围 5 厘米之内的区域除外。
· 如果别人给您注射,也可以注射至您的上臂。
· 每次注射更换部位。
![]() 请勿注射至脆弱、损伤或有瘀伤或疤痕的皮肤上。
请勿注射至脆弱、损伤或有瘀伤或疤痕的皮肤上。

第 6 步:清洁
清洗您的双手。 用酒精擦拭片清洁注射部位。 注射前让皮肤干燥。
![]() 注射前请勿再次触摸注射部位或吹干注射部位。
注射前请勿再次触摸注射部位或吹干注射部位。

第 7 步:拉
手持注射器体中部,针头置于背离您的方向,并拔下针帽。

![]() 请勿将针帽重新盖上。
请勿将针帽重新盖上。 ![]() 请勿触碰针头。 取下针帽后立即注射药物。
请勿触碰针头。 取下针帽后立即注射药物。
第 8 步:捏 如图所示,在注射部位捏起一道皮肤。

第 9 步:插入
将针头以大约 45º 的角度完全插入捏起的皮肤处。

第 10 步:推
放松捏起的部位。 将柱塞杆缓慢且匀速地向下推,直至将注射器排空。 注意:您会感觉到一些阻力,此为正常现象。

第 11 步:释放和拔出 松开拇指并释放柱塞杆,直至针头防护装置包裹针头,然后从注射部位取下注射
器。
若出血,可以用棉球或纱布轻轻按住注射部位。。 ![]() 请勿将针帽重新盖上。
请勿将针帽重新盖上。 ![]() 注射后,请勿摩擦注射部位皮肤。
注射后,请勿摩擦注射部位皮肤。

第 12 步:处置
将注射器和针帽置于防刺穿容器中。 ![]() 请勿将针帽重新盖上。 始终将容器置于儿童不易接触的地方。
请勿将针帽重新盖上。 始终将容器置于儿童不易接触的地方。

![]() 核准日期:2020 年 6 月 17 日 修改日期:
核准日期:2020 年 6 月 17 日 修改日期:
请仔细阅读说明书并在医师指导下使用。
【药品名称】
通用名称:度普利尤单抗注射液 商品名称:达必妥®/ Dupixent® 英文名称:Dupilumab Injection 汉语拼音:Dupuliyou Dankang Zhusheye
【成份】
本品活性成份为度普利尤单抗。度普利尤单抗系采用中国仓鼠卵巢细胞
(CHO-K1)表达制备的靶向白细胞介素-4 受体亚基α(IL-4R-α)的全人单克 隆抗体(IgG4 型),能够抑制 IL-4/IL-13 信号传导。
辅料:组氨酸、盐酸组氨酸、盐酸精氨酸、聚山梨酯 80、醋酸钠、冰醋酸、 蔗糖和注射用水。
【性状】
透明至略带乳白色、无色至淡黄色溶液,无可见颗粒。
【适应症】
本品用于治疗外用处方药控制不佳或不建议使用外用处方药的成人中重度 特应性皮炎。本品可与或不与外用皮质类固醇联合使用。
【规格】
300 mg (2.0 mL)/支(预充式注射器)
【用法用量】
本品应由具有适应症诊断和治疗经验的医疗卫生专业人员处方。 剂量
推荐成人患者使用本品的初始剂量为 600mg (300mg 注射两次),继以每两周
一次给予 300mg,皮下注射给药。
本品可与或不与外用皮质类固醇联合使用。可使用外用钙调神经磷酸酶抑制 剂,但应仅限于问题部位,如面部、颈部、褶皱区域和生殖器部位。
本品治疗特应性皮炎 16 周后无效的患者应考虑停止治疗。一些在初始治疗
中部分应答的患者可能会在 16 周后的继续治疗中获得病情改善。若本品治疗必 须中止,患者仍能成功接受重新治疗。
给药方法
皮下使用。本品通过皮下注射至大腿或腹部,肚脐周围 5 厘米以内的区域除 外。如果由他人进行注射,也可注射于上臂。
对于 600mg 初始剂量,应在不同的注射部位接连注射两次 300mg 本品。 建议每次注射时轮换注射部位。本品不应注射至脆弱、受损或有瘀伤、疤痕
的皮肤上。
在医疗卫生专业人员认可的情况下,患者可以自行注射本品,或者由患者的 看护人员进行给药。在使用前,应根据包装说明书中的使用说明(见文末图示), 对患者和/或看护人员,提供适当的准备和使用本品的培训。
错过用药
如果错过一次用药,应尽快给药。此后,恢复在规定时间内的定期给药。
特殊人群
老年患者(≥65岁)
对于老年患者,不建议调整剂量(见【药代动力学】)。
轻度或中度肾损害患者不需要调整剂量。本品在严重肾损害患者中的数据极 其有限(见【药代动力学】)。
尚无本品在肝功能损害患者中的数据(见【药代动力学】)。
不建议根据体重调整剂量(见【药代动力学】)。
【不良反应】 安全性特征概要
最常见的不良反应是注射部位反应、结膜炎、睑缘炎和口腔疱疹。特应性皮 炎开发项目中报告的血清病/血清病样反应病例非常罕见(见【注意事项】)。
在单药治疗研究中,因不良事件中止治疗的患者比例:安慰剂组为 1.9%, 度普利尤单抗 300mg 每两周一次 (Q2W) 组为 1.9%,度普利尤单抗 300mg 每 一周一次 (QW) 组为 1.5%。在联用外用皮质类固醇 (TCS) 的研究中,因不良事 件中止治疗的患者比例:安慰剂+ TCS 组为 7.6%,度普利尤单抗 300 mg Q2W +
TCS 组为 1.8%,度普利尤单抗 300 mg QW + TCS 组为 2.9%。
不良反应列表
在四项随机、双盲、安慰剂对照研究(分别为 SOLO1, SOLO2, CHRONOS 和 CAFÉ 研究)和一项剂量范围研究(R668-AD-1021 研究)中,评估本品对中 度至重度特应性皮炎患者的安全性。在这 5 项试验中,1689 例受试者接受本品 皮下注射治疗,联合或不联合外用皮质类固醇 (TCS) 。共有 305 例患者接受本 品治疗至少 1 年。
表 1 中列出了按系统器官分类和频率划分的在特应性皮炎临床试验中观察 到的不良反应,使用以下类别:十分常见(≥1/ 10)、常见(≥1/ 100 至<1/10)、偶见 (≥1/ 1,000 至<1/100)、罕见(≥1/ 10,000 至<1/1,000)、十分罕见(<1/10,000)。在每个 频率分组中,不良反应以严重程度递减的顺序呈现。
表 1 特应性皮炎治疗中的不良反应列表
|
MedDRA 系统器官分类 |
频率 |
不良反应 |
|
感染及侵染类疾病 |
常见 |
结膜炎 口腔疱疹 |
|
血液及淋巴系统疾病 |
常见 |
嗜酸性粒细胞增多症 |
|
免疫系统疾病 |
十分罕见 |
血清病/血清病样反应 |
|
各类神经系统疾病 |
常见 |
头痛 |
|
眼器官疾病 |
常见 |
过敏性结膜炎 眼部瘙痒 睑缘炎 |
|
全身性疾病及给药部位各种 反应 |
十分常见 |
注射部位反应 |
特定不良反应描述
罕见本品给药后的血清病/血清病样反应和速发过敏反应病例报告(见【注 意事项】)。
接受本品治疗的特应性皮炎患者出现结膜炎的频率升高。大部分患者的结膜
炎在治疗期间痊愈或好转。(见【注意事项】)。
在 16 周特应性皮炎的单药治疗研究中,度普利尤单抗组疱疹性湿疹报告比 例<1%,安慰剂组报告比例<1%。在 52 周特应性皮炎的度普利尤单抗 + TCS 研 究中,度普利尤单抗 + TCS 组疱疹性湿疹报告比例为 0.2%,安慰剂+ TCS 组报 告比例为 1.9%。
本品治疗患者和安慰剂治疗患者相比,嗜酸粒细胞计数较基线的平均初始增 长更高。在研究治疗期,嗜酸粒细胞计数下降到接近基线水平。
据报告,度普利尤单抗治疗患者治疗中出现的嗜酸粒细胞增多症(≥5,000 细胞/mcL)的比例<1%,安慰剂治疗患者无报告。
在 16 周特应性皮炎的单药治疗研究中,安慰剂组有 1.0%的患者报告严重感 染,度普利尤单抗组有 0.5%的患者报告严重感染。在 52 周特应性皮炎的 CHRONOS 研究中,安慰剂组有 0.6%的患者报告严重感染,度普利尤单抗组有 0.2%的患者报告严重感染。
与所有治疗性蛋白一致,本品也可能具有免疫原性。
抗药抗体 (ADA) 反应通常不会影响本品的暴露、安全性或有效性。 在为期 52 周的研究中,安慰剂组中约 3%的患者、本品组中 2%的患者的抗
药抗体(ADA)反应持续超过 12 周。在这些患者中,安慰剂组有 0.7%的患者,
本品治疗组有 0.2%的患者还具有中和抗体反应,这些反应通常不伴有疗效丧失。
在总体暴露汇总中,少于 0.1%的患者表现出高滴度的 ADA 反应,伴有暴露 和疗效降低。此外,有 1 例血清病患者和 1 例血清病样反应(<0.1%)患者伴有 高滴度 ADA(见【注意事项】)
【禁忌】
对本品活性成分或者其他任何辅料有超敏反应者禁用。
【注意事项】 超敏反应
如果发生全身性超敏反应 (速发型或迟发型) ,应立即停用本品并开始适当 的治疗。本品给药后,特应性皮炎开发项目中报告的血清病/血清病样反应病例
非常罕见。(见【不良反应】)。
蠕虫感染
已知蠕虫感染的患者被排除参加临床研究。本品通过抑制 IL-4/IL-13 信号传 导,可能会影响针对蠕虫感染的免疫应答。原先存在蠕虫感染的患者应在开始使 用本品前进行治疗。如果患者在接受本品治疗期间感染蠕虫,并且对抗蠕虫治疗 无反应,则应停止本品治疗,直至感染消除。
结膜炎相关事件
接受本品治疗的患者,如果发生结膜炎且经标准治疗不能缓解,则应接受眼 科检查(见【不良反应】)。
合并哮喘的特应性皮炎患者
在未咨询医生的情况下,接受本品治疗中度至重度特应性皮炎且合并哮喘的 患者不应调整或停止哮喘治疗方案。停用本品后,应仔细监测合并哮喘患者。
疫苗接种
本品给药时,不得同时给予活疫苗和减毒活疫苗,因为尚未确定此类操作的 临床安全性和疗效。已评估了对 TdaP 疫苗 (破伤风、白喉和百日咳三联疫苗) 和 脑膜炎球菌多糖疫苗的免疫应答(见【药物相互作用】)。建议在本品治疗前,患 者应已根据当前免疫指南完成活疫苗和减毒活疫苗接种。
钠含量
本品每 300mg 剂量含钠低于 1mmol (23mg),即基本“不含钠”。
对驾驶和操作机器能力的影响
本品对驾驶或操作机器能力无影响或可以忽略不计。
配伍禁忌
在无配伍禁忌研究的情况下,本品不得与其他药品混和。
【孕妇及哺乳期妇女用药】 妊娠
本品在孕妇使用的数据非常有限。就生殖毒性而言,动物研究并未提示直接 或间接的有害影响(见【药理毒理】)。本品是重组人 IgG4 单克隆抗体,已知人 IgG 抗体可穿过胎盘屏障,因此本品可能从母体传输至发育中的胎儿。只有证明 潜在获益大于胎儿潜在风险时,才可在妊娠期间使用本品。
哺乳
尚不清楚本品是否在人乳中排泄或摄入后全身吸收,但已知母体 IgG 存在 于母乳中。做出是否停止母乳喂养或停止本品治疗的决定必须考虑母乳喂养对儿 童的益处以及治疗对母亲的益处。
生育力
动物研究未显示生育力受损(见【药理毒理】)。
【儿童用药】
本品在中国 18 岁以下特应性皮炎儿童患者的安全性和有效性尚未确立。在
国外本品已完成用于治疗 12-17 岁特应性皮炎儿童患者的临床试验,包括患者药 代动力学(PK)研究和多中心随机对照的有效性/安全性研究。
【老年用药】
群体药代动力学分析未发现年龄对本品全身暴露有任何具有临床意义的影 响。见【药代动力学】
【药物相互作用】
在使用本品每周治疗一次,共 16 周的方案治疗特应性皮炎患者的研究中, 评估了疫苗的免疫应答。经 12 周本品给药后,患者接种 Tdap 疫苗 (T 细胞依赖 性) 和脑膜炎球菌多糖疫苗 (T 细胞非依赖性) ,并在 4 周后评估免疫应答。本 品治疗组和安慰剂组患者对破伤风疫苗和脑膜炎球菌多糖疫苗的抗体反应相似。 在研究中未观察到非活疫苗和本品之间的不良相互作用。
因此,接受本品治疗的患者可同时接种灭活或非活疫苗。关于活疫苗的信息, 见【注意事项】。
在一项特应性皮炎患者的临床研究中,评估了本品对 CYP 底物药代动力学 (PK)的影响。该研究收集的数据并未显示本品对 CYP1A2、CYP3A、CYP2C19、 CYP2D6 或 CYP2C9 活性具有临床相关的影响。
【药物过量】
本品用药过量无特殊治疗方法。如果出现药物过量,应监测患者是否有任何 不良反应体征或症状,并立即采取适当的对症治疗。
【临床试验】
在 全球 三项 关 键性随 机 、双盲 、 安慰剂 对 照研究 (SOLO1 , SOLO2 和 CHRONOS)中评估了本品单药治疗以及联合外用皮质类固醇的疗效和安全性, 共有 2119 例年龄在 18 岁及以上的中度至重度特应性皮炎(AD)患者参与,中至 重度特应性皮炎定义为研究者总体评估(IGA)评分≥3,湿疹面积和严重程度指数
(EASI)评分≥16,最低受累及体表面积(BSA)≥10%。入选至这三项研究符合条件 的患者既往使用外用药物疗效不足。
在所有三项研究中,患者 1) 在第 1 天接受初始剂量为 600mg 的度普利尤单 抗 (300mg,两次注射) ,然后每两周一次 300mg (Q2W) ;2) 在第 1 天接受初 始剂量为 600mg 的度普利尤单抗,随后每周一次 300mg (QW) ;或 3) 接受相匹 配的安慰剂。在所有研究中,本品通过皮下 (SC) 注射给药。如果需要控制无法 耐受的特应性皮炎症状,则允许患者根据研究者的判断接受补救治疗 (包括更高 效的外用皮质类固醇或全身性免疫抑制剂)。接受补救治疗的患者视为非应答者。
SOLO1 研究入选了 671 例患者 (224 例接受安慰剂治疗,224 例接受度普利 尤单抗 300mg Q2W 治疗,223 例接受度普利尤单抗 300mg QW 治疗) ,治疗周 期为 16 周。
SOLO2 研究入选了 708 例患者 (236 例接受安慰剂治疗,233 例接受度普利 尤单抗 300mg Q2W 治疗,239 例接受度普利尤单抗 300mg QW 治疗),治疗周 期为 16 周。
CHRONOS 研究入选了 740 例患者(315 例接受安慰剂+ TCS 治疗,106 例接 受度普利尤单抗 300mg Q2W + TCS 治疗,319 例接受度普利尤单抗 300mg QW
+ TCS 治疗),治疗周期为 52 周。接受本品或安慰剂联合使用 TCS 的患者从基线 开始使用标准化方案。还允许患者使用外用钙调神经磷酸酶抑制剂(TCI)。
终点
在所有三项关键性研究中,并列主要终点是自基线至第 16 周时 IGA 0 或 1(“清除”或“几乎清除”)且 0-4 IGA 评分至少下降 2 分的患者比例、EASI 至少改 善 75%(EASI-75)的患者比例。其他评估结果包括自基线至第 16 周 EASI 至少改 善 50%和 90%(分别为 EASI-50 和 EASI-90)的患者比例、由峰值瘙痒数字评估量 表(NRS)测量的瘙痒减少程度以及特应性皮炎评分(SCORAD)变化百分比。其他 次要终点包括患者湿疹自我评价(POEM)、皮肤病生活质量指数(DLQI)和医院焦 虑抑郁量表(HADS)评分自基线至第 16 周的平均变化。在 CHRONOS 研究中, 还在第 52 周时评估疗效。
基线特征
在单药治疗研究中(SOLO1 和 SOLO2),所有治疗组的平均年龄为 38.3 岁, 平均体重为 76.9kg,女性占 42.1%,白种人占 68.1%,亚洲人占 21.8%,黑人占 6.8%。在这些研究中,51.6%的患者基线 IGA 评分为 3 分(中度 AD),48.3%的患 者基线 IGA 评分为 4(重度 AD),32.4%的患者既往接受全身性免疫抑制剂治疗。 基线平均 EASI 评分为 33.0,基线瘙痒 NRS 周平均值为 7.4,基线平均 SCORAD 评分为 67.8,基线平均 POEM 评分为 20.5,基线平均 DLQI 为 15.0,基线平均 HADS 总评分为 13.3。
在联合 TCS 的研究(CHRONOS)中,所有治疗组的平均年龄为 37.1 岁,平均 体重为 74.5kg,女性占 39.7%,白种人占 66.2%,亚洲人占 27.2%,黑人占 4.6%。 在这项研究中,53.1%的患者基线 IGA 评分为 3 分,46.9%的患者基线 IGA 评分 为 4 分,33.6%的患者既往接受全身性免疫抑制剂治疗。基线平均 EASI 评分为 32.5,基线周瘙痒 NRS 为 7.3,基线平均 SCORAD 评分为 66.4,基线平均 POEM 评分为 20.1,基线平均 DLQI 为 14.5,基线平均 HADS 总评分为 12.7。
临床应答
16 周单药治疗研究(SOLO1 和 SOLO2)
在 SOLO1 和 SOLO2 研究中,从基线至第 16 周,与安慰剂组相比,随机分 配至本品治疗的患者显著有更大比例患者实现 IGA 0 或 1 应答、EASI-75 和/或 瘙痒 NRS 改善≥ 4 分(见表 2)。
与安慰剂组相比,随机分配至本品治疗的患者显著有更大比例患者实现瘙痒 NRS 迅速改善(定义为早在第 2 周改善≥4 分;p <0.01),在整个治疗期,瘙痒 NRS 应答患者比例继续增加。瘙痒 NRS 改善伴随特应性皮炎客观体征的改善。
图 1 和图 2 分别显示了自基线至第 16 周时的 EASI 平均变化百分比和 NRS
平均变化百分比。
表 2 第 16 周本品单药治疗的疗效结果(FAS)
|
|
SOLO1 (FAS)a |
SOLO2 (FAS)a |
||||
|
|
安慰剂 |
度普利尤 单抗 300 mg Q2W |
度普利尤 单抗 300 mg QW |
安慰剂 |
度普利尤 单抗 300 mg Q2W |
度普利尤 单抗 300 mg QW |
|
随机分组的患者 数量 |
224 |
224 |
223 |
236 |
233 |
239 |
|
IGA 0 或 1b,应 答者百分比 c |
10.3% |
37.9%e |
37.2%e |
8.5% |
36.1%e |
36.4%e |
|
EASI-50,应答 者百分比 c |
24.6% |
68.8%e |
61.0%e |
22.0% |
65.2%e |
61.1%e |
|
EASI-75,应答 者百分比 c |
14.7% |
51.3%e |
52.5%e |
11.9% |
44.2%e |
48.1%e |
|
EASI-90,应答 者百分比 c |
7.6% |
35.7%e |
33.2%e |
7.2% |
30.0%e |
30.5%e |
|
EASI,自基线的 |
-37.6% |
-72.3%e |
-72.0%e |
-30.9% |
-67.1%e |
-69.1%e |
|
LS 平均变化百 |
(3.28) |
(2.63) |
(2.56) |
(2.97) |
(2.52) |
(2.49) |
|
分比(+/-SE) |
||||||
|
SCORAD,自基 |
-29.0% |
-57.7%e |
-57.0%e |
-19.7% |
-51.1%e |
-53.5%e |
|
线的 LS 平均变 |
(3.21) |
(2.11) |
(2.11) |
(2.52) |
(2.02) |
(2.03) |
|
化百分比(+/-SE) |
||||||
|
瘙痒 NRS,自基 |
-26.1% |
-51.0%e |
-48.9%e |
-15.4% |
-44.3%e |
-48.3%e |
|
线的 LS 平均变 化百分比(+/-SE) |
(3.02) |
(2.50) |
(2.60) |
(2.98) |
(2.28) |
(2.35) |
|
基线瘙痒 NRS 评分≥4 的患者数 量 |
212 |
213 |
201 |
221 |
225 |
228 |
|
瘙痒 NRS (改 善>4 分),应答 者百分比 c,d |
12.3% |
40.8%e |
40.3%e |
9.5% |
36.0%e |
39.0%e |
LS =最小二乘法;SE =标准误
a 全分析集(FAS)包括所有随机分组的患者。
b 应答者定义为 IGA 0 或 1(“清除”或“几乎清除”)且 0-4 IGA 评分降低≥ 2 分的患者。
c 接受过补救治疗或数据缺失的患者视为非应答者。
d 第 2 周时,相比于安慰剂组,度普利尤单抗组中显著有更多患者瘙痒 NRS 评分改善≥4 分(P
<0.01)。
e p 值<0.0001。
图 1 SOLO1a 和 SOLO2a(FAS)b 研究中 EASI 自基线的平均变化百分比
LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 全分析集(FAS)包括所有随机分组的患者。
图 2 SOLO1a 和 SOLO2a(FAS)b 研究中 NRS 自基线的平均变化百分比

LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 全分析集(FAS)包括所有随机分组的患者。
SOLO1 和 SOLO2 亚组(体重、年龄、性别、种族和背景治疗,包括免疫抑 制剂)的治疗效果与总体研究人群结果一致。
52 周联合 TCS 的研究(CHRONOS)
从基线至第 16 周及第 52 周,与安慰剂+TCS 组相比,随机分配至度普利尤 单抗 300 mg Q2W + TCS 治疗的患者显著有更大比例患者实现 IGA 0 或 1 应答, EASI-75 和/或瘙痒 NRS 改善≥ 4 分(见表 3)。
与安慰剂+TCS 组相比,随机分配至度普利尤单抗+TCS 治疗的患者显著有 更大比例患者实现瘙痒 NRS 迅速改善(定义为早在第 2 周改善≥4 分; p <0.05),在 整个治疗期,瘙痒 NRS 应答患者的比例继续增加。瘙痒 NRS 改善伴随特应性皮 炎客观体征的改善。
图 3 和图 4 分别显示了在 CHRONOS 研究中直至第 52 周时的 EASI 较基线 平均变化百分比和 NRS 较基线平均变化百分比。
表 3 CHRONOS 研究中本品联合使用 TCSa 第 16 周和第 52 周的疗效结果
 第 16 周(FAS)b 第 52 周(第 52 周的 FAS)b
第 16 周(FAS)b 第 52 周(第 52 周的 FAS)b
|
|
安慰 剂 + TCS |
度普利尤 单抗 300 mg Q2W + TCS |
度普利尤 单抗 300 mg QW + TCS |
安慰 剂 + TCS |
度普利尤 单抗 300 mg Q2W + TCS |
度普利尤 单抗 300 mg QW + TCS |
|
随机分组的患者数量 |
315 |
106 |
319 |
264 |
89 |
270 |
|
IGA 0 或 1c,应答者百 |
12.4% |
38.7%f |
39.2%f |
12.5% |
36.0%f |
40.0%f |
|
分比 d |
|
|
|
|
|
|
|
EASI-50,应答者百分 |
37.5% |
80.2%f |
78.1%f |
29.9% |
78.7%f |
70.0%f |
|
比 d |
|
|
|
|
|
|
|
EASI-75,应答者百分 |
23.2% |
68.9%f |
63.9%f |
21.6% |
65.2%f |
64.1%f |
|
比 d |
|
|
|
|
|
|
|
EASI-90,应答者百分 |
11.1% |
39.6%f |
43.3%f |
15.5% |
50.6%f |
50.7%f |
|
比 d |
|
|
|
|
|
|
|
EASI,自基线的 LS |
- |
-80.5%f |
-81.5%f |
- |
-84.9%g |
-87.8%h |
|
平均变化百分比(+/- |
48.4% |
(6.34) |
(5.78) |
60.9% |
(6.73) |
(6.19) |
|
SE) |
(3.82) |
|
|
(4.29) |
|
|
|
SCORAD,自基线的 |
- |
-63.9%f |
-65.9%f |
- |
-69.7%f |
-70.4%f |
|
LS 平均变化百分比 |
36.2% |
(2.52) |
(1.49) |
47.3% |
(3.06) |
(1.72) |
|
(+/-SE) |
(1.66) |
|
|
(2.18) |
|
|
|
瘙痒 NRS,自基线的 |
- |
-56.6%f |
-57.1%f |
- |
-57.0%i |
-56.5%f |
|
LS 平均变化百分比 |
30.3% |
(3.95) |
(2.11) |
31.7% |
(6.17) |
(3.26) |
|
(+/-SE) |
(2.36) |
|
|
(3.95) |
|
|
|
基线瘙痒 NRS 评分≥4 的患者数量 |
299 |
102 |
295 |
249 |
86 |
249 |
|
瘙痒 NRS(改善>4 |
19.7% |
58.8%f |
50.8%f |
12.9% |
51.2%f |
39.0%f |
|
分),应答者百分比 d, e |
|
|
|
|
|
|
LS =最小二乘法;SE =标准误
a 所有患者均接受外用皮质类固醇背景治疗,并允许患者使用外用钙调神经磷酸酶抑制剂。 b 全分析集(FAS)包括所有随机分组的患者。第 52 周的 FAS 包括所有在主要分析截止日期之 前至少一年随机分组的患者。
c 应答者定义为 IGA 0 或 1(“清除”或“几乎清除”)且 0-4 IGA 评分降低≥ 2 分的患者。
d 接受过补救治疗或数据缺失的患者视为非应答者。
e 第 2 周时,相比于安慰剂组,度普利尤单抗组中显著有更多患者瘙痒 NRS 评分改善≥4 分
(P <0.05)。
f p 值<0.0001 g p 值= 0.0015 h p 值= 0.0003 i p 值= 0.0005
图 3 CHRONOSa 研究中(第 52 周的 FAS)bEASI 自基线的平均变化百分比

LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 第 52 周的 FAS 包括所有在主要分析截止日期之前至少一年随机分组的患者。
图 4 CHRONOSa 研究中(第 52 周的 FAS)b NRS 自基线的平均变化百分比
CHRONOS

LS =最小二乘法
a 在疗效终点的主要分析中,接受补救治疗或缺失数据的患者视为非应答者。
b 第 52 周的 FAS 包括所有在主要分析截止日期之前至少一年随机分组的患者。
CHRONOS 亚组(体重、年龄、性别、种族和背景治疗,包括免疫抑制剂)的 治疗效果与总体研究人群结果一致。
(CAFE 研究)
CAFE 研究评估了成年 AD 患者使用本品相比安慰剂(联合使用 TCS)治疗 16 周期间的疗效,这些患者口服环孢素不能充分控制病情、或不耐受环孢素或当前 使用环孢素为禁忌或医学上不适用。
共有 325 例患者入选,其中 210 例患者既往暴露于环孢素,115 例患者因在 医学上不适用环孢素治疗从未暴露于环孢素。平均年龄为 38.4 岁,女性占 38.8%, 基线平均 EASI 评分为 33.1,平均 BSA 为 55.7,基线瘙痒 NRS 周平均值为 6.4, 基线平均 SCORAD 评分为 67.2,基线平均 DLQI 为 13.8 。
主要终点是第 16 周时实现 EASI-75 的患者比例。 表 4 总结了 16 周 CAFE 研究的主要和次要终点。
表 4 CAFE 研究中主要和次要终点结果
|
|
安慰剂 + TCS |
度普利尤单抗 300 mg Q2W + TCS |
度普利尤单抗 300 mg QW+TCS |
|
随机分组的患者数量 |
108 |
107 |
110 |
|
EASI-75, 应答者百分比 |
29.6% |
62.6% |
59.1% |
|
EASI, 自基线的 LS 平均变化 |
-46.6 |
-79.8 |
-78.2 |
|
百分比(+/-SE) |
(2.76) |
(2.59) |
(2.55) |
|
瘙痒 NRS,自基线的平均变化 |
-25.4% |
-53.9% |
-51.7% |
|
百分比(+/-SE) |
(3.39) |
(3.14) |
(3.09) |
|
SCORAD, 自基线的 LS 平均 |
-29.5% |
-62.4% |
-58.3% |
|
变化百分比(+/-SE) |
(2.55) |
(2.48) |
(2.45) |
|
DLQI, 自基线的 LS 平均变化 |
-4.5 |
-9.5 |
-8.8 |
|
百分比(+/-SE) |
(0.49) |
(0.46) |
(0.45) |
(p 值均<0.0001)
在 52 周 CHRONOS 研究中,类似于 CAFE 研究人群的患者亚组,接受度普 利尤单抗 300mg Q2W 治疗的患者有 69.6%在第 16 周时达到 EASI-75,而接受 安慰剂治疗的患者有 18.0%达到 EASI-75;第 52 周时,接受度普利尤单抗 300mg Q2W 治疗的患者有 52.4%达到 EASI-75,而接受安慰剂治疗的患者有 18.6%达到 EASI-75。在该亚集中,度普利尤单抗 300mg Q2W 治疗组和安慰剂治疗组瘙痒 NRS 自基线变化的百分比在第 16 周分别为-51.4% 和 -30.2%,在第 52 周分别 为-54.8%和 -30.9%。
疗效的维持和持久性(SOLO CONTINUE 研究)
为了评估疗效的维持和持久性,在 SOLO1 和 SOLO2 研究中接受本品治疗 16 周并实现 IGA 0 或 1 或 EASI-75 的受试者在 SOLO CONTINUE 研究中再随机 分配,再进行额外的 36 周本品或安慰剂治疗,以达到为期 52 周的累积研究治
疗。在第 51 或 52 周评估终点。
并列主要终点是 EASI 评分相对 SOLO1 和 SOLO2 研究基线的百分比变化
在基线(第 0 周)和第 36 周之间的差异,以及基线时 EASI-75 患者中第 36 周时仍 达 EASI-75 患者的百分比。
继续接受 SOLO1 和 SOLO2 研究中相同剂量方案治疗的患者(300 mg Q2W 或 300 mg QW)显示维持临床反应的最佳效果,而其他剂量方案的疗效以剂量依 赖性方式下降。
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
†P<0.05, *P<0.01, **P<0.001, ***P≤0.0001
在 SOLO CONTINUE 研究中,观察到治疗中出现的 ADA 阳性率随着给药 间隔增加而升高的趋势。治疗中出现的 ADA:QW:1.2%、Q2W:4.3%、Q4W: 6.0%、Q8W:11.7%。持续超过 12 周的 ADA 反应:QW:0.0%、Q2W:1.4%、 Q4W:0.0%、Q8W:2.6%。
在单药治疗研究(SOLO1 和 SOLO2)中,第 16 周时,与安慰剂组比较,度普 利尤单抗 300 mg Q2W 和 300 mg QW 组分别显著改善了患者报告的症状以及 AD 对睡眠和健康相关生活质量的影响(由 POEM 和 DLQI 总评分衡量)。与安慰 剂组相比,从基线至第 16 周,本品给药组中有更多比例患者 POEM 和 DLQI 总 分出现有临床意义的降低(每种评分均定义为改善≥4 分)。此外,与安慰剂组相比, 第 16 周时,度普利尤单抗组由 HADS 总评分衡量的焦虑和抑郁症状显著减少。 在基线 HADS 焦虑或 HADS 抑郁子量表评分≥8 分(焦虑或抑郁的界值)的患者子
集中,与安慰剂组相比,第 16 周时度普利尤单抗组中有更多比例的患者达到
HADS 焦虑和 HADS 抑郁评分<8(见表 6)。
表 6 第 16 周时度普利尤单抗单药治疗的其他次要终点结果
|
|
单药治疗 |
|||||
|
|
SOLO1 第 16 周时 |
SOLO2 第 16 周时 |
||||
|
|
安慰 |
度普利尤单抗 |
度普利尤单 |
安慰 |
度普利尤单抗 |
度普利尤单 |
|
剂 |
300 mg Q2W |
抗 |
剂 |
300 mg Q2W |
抗 |
|
|
300 mg QW |
300 mg QW |
|||||
|
随机分组的患者数量 |
224 |
224 |
223 |
236 |
233 |
239 |
|
DLQI,自基线的 LS 平 |
-5.3 |
-9.3a |
-9.0a |
-3.6 |
-9.3a |
-9.5a |
|
均变化 (SE) |
(0.50) |
(0.40) |
(0.40) |
(0.50) |
(0.38) |
(0.39) |
|
POEM, 自基线的 LS |
-5.1 |
-11.6a |
-11.0a |
-3.3 |
-10.2a |
-11.3a |
|
平均变化 (SE) |
(0.67) |
(0.49) |
(0.50) |
(0.55) |
(0.49) |
(0.52) |
|
HADS,自基线的 LS |
-3.0 |
-5.2b |
-5.2b |
-0.8 |
-5.1a |
-5.8a |
|
平均变化, (SE) |
(0.65) |
(0.54) |
(0.51) |
(0.44) |
(0.39) |
(0.38) |
|
|
||||||
|
基线时 DLQI ≥4 的患 者数量 |
213 |
209 |
209 |
225 |
223 |
234 |
|
DLQI(改善≥4 分), 应 答者百分比 |
30.5% |
64.1%a |
58.4%a |
27.6% |
73.1%a |
62.0%a |
|
|
||||||
|
基线时 POEM ≥4 的 患者数量 |
223 |
222 |
222 |
234 |
233 |
239 |
|
POEM(改善≥4 分), 应 答者百分比 |
26.9% |
67.6%a |
63.1%a |
24.4% |
71.7%a |
64.0%a |
|
|
||||||
|
基线时 HADS 焦虑评 分≥8 或 HADS 抑郁评 分 ≥8 的患者数量 |
97 |
100 |
102 |
115 |
129 |
136 |
|
达到 HADS 焦虑和 HADS 抑郁评分<8 的 患者,% |
12.4% |
41.0%a |
36.3%b |
6.1% |
39.5%a |
41.2%a |
LS = 最小二乘法; SE =标准误
a p 值<0.0001 b p 值<0.001
在联合 TCS 的研究(CHRONOS)中,第 52 周时,与安慰剂+TCS 组比较,度 普利尤单抗 300 mg Q2W+TCS 和度普利尤单抗 300 mg QW+TCS 组分别显著改 善了患者报告的症状以及 AD 对睡眠和健康相关生活质量的影响(由 POEM 和 DLQI 总评分衡量)。与安慰剂组相比,从基线至第 52 周,度普利尤单抗 300 mg Q2W+TCS 和 300 mg QW+TCS 给药组中,有更多比例患者 POEM 和 DLQI 总分 出现有临床意义的降低(每种评分均定义为改善≥4 分)。此外,与安慰剂+TCS 组 相比,第 52 周时,度普利尤单抗 300 mg Q2W+TCS 和 300 mg QW+TCS 组由
HADS 总评分衡量的焦虑和抑郁症状显著减少。在基线时具有 HADS 焦虑或 HADS 抑郁子量表评分≥8 分(焦虑或抑郁的界值)的患者子集的事后分析中,与安 慰剂+ TCS 组相比,第 52 周时度普利尤单抗 300 mg Q2W + TCS 和 300mg QW
+ TCS 组中有更多比例患者达到 HADS-焦虑和 HADS-抑郁评分<8(参见表 7)。
表 7 CHRONOS 研究中本品伴随 TCS 治疗第 16 周和第 52 周时的其他次要终点结果
|
|
伴随使用 TCS |
|||||
|
|
CHRONOS 第 16 周时 |
CHRONOS 第 52 周时 |
||||
|
|
安慰剂 |
度普利尤单 |
度普利尤单 |
安慰剂 |
度普利尤单 |
度普利尤 |
|
抗 300 mg |
抗 300 mg |
+TCS |
抗 300 mg |
单抗 300 |
||
|
Q2W +TCS |
QW +TCS |
Q2W+ TCS |
mg QW+ |
|||
|
TCS |
||||||
|
随机分组的患者数量 |
315 |
106 |
319 |
264 |
89 |
270 |
|
DLQI, 自基线的 LS |
-5.8 |
-10.0a |
-10.7a |
-7.2 |
-11.4a |
-11.1a |
|
平均变化 (SE) |
(0.34) |
(0.50) |
(0.31) |
(0.40) |
(0.57) |
(0.36) |
|
POEM, 自基线的 LS |
-5.3 |
-12.7a |
-12.9a |
-7.0 |
-14.2a |
-13.2a |
|
平均变化 (SE) |
(0.41) |
(0.64) |
(0.37) |
(0.57) |
(0.78) |
(0.45) |
|
HADS, 自基线的 LS |
-4.0 |
-4.9 |
-5.4c |
-3.8 |
-5.5c |
-5.9b |
|
平均变化 (SE) |
(0.37) |
(0.58) |
(0.35) |
(0.47) |
(0.71) |
(0.42) |
|
|
||||||
|
基线时 DLQI ≥4 的患 者数量 |
300 |
100 |
311 |
254 |
85 |
264 |
|
DLQI (改善≥4 分), 应 答者百分比 |
43.0% |
81.0 %a |
74.3 %a |
30.3% |
80.0 %a |
63.3 %a |
|
|
||||||
|
基线时 POEM ≥4 的患 者数量 |
312 |
106 |
318 |
261 |
89 |
269 |
|
POEM (改善≥4 分), 应 答者百分比 |
36.9% |
77.4 %a |
77.4 %a |
26.1% |
76.4 %a |
64.7 %a |
|
|
||||||
|
基线时 HADS 焦虑 评分≥8 或 HADS 抑郁 评分 ≥8 的患者数量 |
148 |
59 |
154 |
133 |
53 |
138 |
|
达到 HADS 焦虑和 HADS 抑郁评分<8 的 患者百分比 |
26.4% |
47.5%c |
47.4%b |
18.0% |
43.4%b |
44.9%a |
LS =最小二乘法; SE =标准误
a p 值<0.0001 b p 值<0.001 c p 值<0.05
【药理毒理】
药理作用
作用机制
度普利尤单抗是一种全人单克隆抗体(IgG4 型),可通过与白介素-4(IL- 4)和白介素-13(IL-13)受体复合物共享的 IL-4Rα 亚单位特异性结合而抑制 IL-
4 和 IL-13 的信号传导。度普利尤单抗通过 I 型受体抑制 IL-4 信号传导,并通过
II 型受体抑制 IL-4 和 IL-13 信号传导。 炎症是哮喘和特应性皮炎发病机理的重要组成部分。炎症涉及可表达 IL-
4Rα 的多种细胞类型(如:肥大细胞、嗜酸粒细胞、巨噬细胞、淋巴细胞、上皮 细胞、杯状细胞)和炎性介质(如:组胺、类花生酸、白三烯、细胞因子、趋化 因子)。利用度普利尤单抗阻断 IL-4Rα,可抑制 IL-4 和 IL-13 细胞因子诱导的炎 性反应,包括:促炎细胞因子、趋化因子、一氧化氮和 IgE 的释放;但度普利尤 单抗对哮喘的作用机制尚未明确。
毒理研究
遗传毒性 尚未开展动物试验评价度普利尤单抗的致突变性。 生殖毒性
性成熟小鼠皮下注射剂量高达 200 mg/kg/周的抗 IL-4Rα 同源抗体,未见 对生殖器官、月经周期长度或精子分析等生育力指标的影响。
围产期毒性研究中,从器官形成开始至分娩,妊娠食蟹猴皮下注射抗 IL-4Rα 同源抗体 100 mg/kg/周(以 mg/kg 计,为最大推荐人用剂量 MRHD 的 10 倍)。 在出生至 6 月龄胎仔中未见胚胎-胎仔毒性或畸形,或对形态学、功能或免疫学 发育的治疗相关不良影响。
致癌性 尚未开展动物试验评价度普利尤单抗的致癌性。
【药代动力学】 吸收
单次皮下(SC)注射 75-600 mg 剂量的本品后,达到血清峰浓度的中位时间为 3-7 天。通过群体药代动力学(PK)分析确定,皮下注射给药后,估计本品的绝对 生物利用度为 64%。
起始给药剂量 600mg,随后每两周给药 300mg,第 16 周达到稳态浓度。在
所有临床试验中,每两周给予 300 mg 剂量,稳态谷浓度平均值±SD 范围为 73.3
±40.0 mcg/mL 至 79.9±41.4 mcg/mL。
分布
通过群体 PK 分析估计本品的分布容积大约为 4.6L,表明本品主要分布在血 管系统中。
生物转化
未进行特定的代谢研究,因为本品是一种蛋白质。预期本品会降解为小肽和 单个氨基酸。
消除
本品可通过平行线性和非线性途径消除。在较高浓度下,本品主要通过非饱 和的蛋白水解途径进行消除,而在较低浓度时,非线性饱和 IL-4Rα 靶点介导的 消除占优势。在末次稳态剂量给药后,通过群体 PK 分析估计,300mg Q2W 方 案度普利尤单抗浓度降至低于检测下限的中位时间为 10 周,300mg QW 方案为 13 周。
线性/非线性
由于非线性清除,以浓度-时间曲线下面积测量的本品暴露随着单次皮下注 射剂量的增加(75mg-600mg)以高于剂量增加比例的方式增加。
特殊人群
通过群体 PK 分析确定,未发现性别对本品全身暴露有任何临床有意义的影 响。
在 2 期剂量范围研究或 3 期安慰剂对照研究中,共有 1472 例特应性皮炎患
者接受本品治疗,其中共有 67 例患者年龄在 65 岁或以上。虽然老年患者和年轻 特应性皮炎成人患者之间的安全性或有效性没有差异,65 岁及以上的患者人数 不足以确定是否与年轻患者的反应不同。
通过群体 PK 分析确定,未发现年龄对本品全身暴露有任何临床有意义的影 响。然而,本分析中仅有 61 例 65 岁以上的患者。
通过群体 PK 分析确定,未发现种族对本品全身暴露有任何临床有意义的影 响。
作为单克隆抗体,本品预期不会发生显著的肝脏消除。未进行临床研究评估 肝损害对本品药代动力学的影响。
作为单克隆抗体,本品预期不会发生显著的肾脏消除。未进行临床研究评估 肾损害对本品药代动力学的影响。群体 PK 分析未发现轻度或中度肾损害对本品 全身暴露有临床意义的影响。本品在严重肾损害患者中的资料非常有限。
在体重较高的受试者中本品谷浓度较低,对疗效未产生有意义的影响。
在中国健康受试者中单次皮下注射本品 200 mg、300 mg 和 600 mg 后,达到 血清峰浓度的中位时间为 7-8.5 天。血清中功能性度普利尤单抗的平均最大浓度
(Cmax)分别为 25.4、37.2 和 77.3 mg/L,随剂量近似成比例增加。AUC 和 AUClast 以大于剂量比例的方式增加,AUC 的平均值分别为 425,807 和 2150 mg·day/L,AUClast 的平均值分别为 402, 792 和 2110 mg·day/L。
【贮藏】
于 2~8℃避光、密封贮藏,避免冷冻。
运输过程中:冷藏储存(2~8℃),注射器应保存在包装盒内,不能进行冷 冻。
患者使用时:通常情况下需冷藏储存(2~8℃)。如有特殊需要,可在常温
(<25℃)条件下储存 14 天,须避光保存,且不可再返回冷藏储存(2~8℃)。 如果在 14 天内没有使用或储存温度超过 25℃应丢弃。
【包装】
2mL 溶液装于带有一个固定不锈钢针头的预充式注射器(1 型透明玻璃)中, 预充式注射器带有针头防护的安全装置。
包装规格为 2 支/盒。
【有效期】
36 个月。
【执行标准】JS20190073
【批准文号】进口药品注册证号:S20200017
【药品上市许可持有人】
名称: Sanofi-aventis groupe
注册地址:54, rue La Boétie, 75008 Paris, France
【生产企业】
企业名称:Sanofi Winthrop Industrie
生产地址:1051 Boulevard Industriel, 76580 Le Trait, France
【境内联系机构】 名称:赛诺菲(中国)投资有限公司 地址:北京市朝阳区建国路 112 号 7 层 联系方式:800(400)-820-8884
使用本品之前请仔细阅读该说明书的全部内容,因为该说明书中包含重要的 用药信息。
图 1 带有针头防护装置的预充式注射器的示意图

重要信息
该装置为一次性使用的预充式注射器,含有 300mg 用于注射至皮肤下的度 普利尤单抗(皮下注射)。除非您的医疗卫生专业人员对您进行过培训,否则您不 得尝试自行或给他人注射本品。
· 使用注射器之前,请仔细阅读所有使用说明。
· 咨询您的医疗卫生专业人员您需要多长时间注射一次药物。
· 要求您的医疗卫生专业人员在首次注射前向您展示正确使用注射器的方 法。
· 每次注射更换注射部位。
· 如果注射器掉落在坚硬的表面上或损坏,请勿使用注射器。
· 如果针帽缺失或未牢固连接,请勿使用注射器。
· 在准备好注射之前,请勿触碰柱塞杆。
· 请勿透过衣服注射。
· 请勿去除注射器中的气泡。
· 为防止意外的针头损伤,每个预充式注射器都有一个针头防护装置,在 您注射后,针头防护装置会自动启动以覆盖针头。
· 请勿回拉柱塞杆。
· 请勿重复使用注射器。
如何存储度普利尤单抗
· 将注射器置于儿童无法接触的地方。
· 将未使用的注射器存放于原包装盒内,并在 2℃-8℃条件下存放于冰箱 中。
· 请勿将度普利尤单抗于室温(<25℃)下保存超过 14 天。如果您需要从冰 箱中永久取出包装盒,请在外包装盒上的空白处记录取出日期,并在 14 天内使用度普利尤单抗。
· 任何时候请勿振摇注射器。
· 请勿加热注射器。
· 请勿冷冻注射器。
· 请勿将注射器置于阳光直射的地方。
第 1 步:取出注射器 握住注射器主体中部,从包装盒中取出注射器。 ![]() 在准备好注射之前,请勿拔下针帽。
在准备好注射之前,请勿拔下针帽。
![]() 如果注射器掉落到坚硬的表面或损坏,请勿使用。
如果注射器掉落到坚硬的表面或损坏,请勿使用。

第 2 步:准备
确保您有以下几件物品:
· 度普利尤单抗预充式注射器
· 1 块酒精擦拭片*
· 1 个棉球或纱布*
· 防刺穿容器*(请参阅步骤 12)
*不包含在包装盒内的物品
看标签:
· 检查有效期限。
· 检查是否为正确的产品和剂量。
![]() 如果有效期限已过,请勿使用注射器。
如果有效期限已过,请勿使用注射器。 ![]() 请勿将本品于室温下放置超过 14 天。
请勿将本品于室温下放置超过 14 天。

第 3 步:检查
通过注射器的观察窗观察药物:检查液体是否为透明无色至浅黄色。
注意:您可能会看到气泡,此为正常现象。
![]() 如果液体变色或浑浊,或含有块状物或颗粒,请勿使用注射器。
如果液体变色或浑浊,或含有块状物或颗粒,请勿使用注射器。

第 4 步:等待 45 分钟
 将注射器置于平坦表面上至少 45 分钟,使其自然达到室温。
将注射器置于平坦表面上至少 45 分钟,使其自然达到室温。 ![]() 请勿加热注射器。
请勿加热注射器。 ![]() 请勿将注射器置于阳光直射的地方。
请勿将注射器置于阳光直射的地方。 ![]() 请勿将度普利尤单抗于室温下放置超过 14 天。
请勿将度普利尤单抗于室温下放置超过 14 天。
第 5 步:选择 选择注射部位。
· 可以注射至大腿或腹部(肚子),肚脐周围 5 厘米之内的区域除外。
· 如果别人给您注射,也可以注射至您的上臂。
· 每次注射更换部位。
![]() 请勿注射至脆弱、损伤或有瘀伤或疤痕的皮肤上。
请勿注射至脆弱、损伤或有瘀伤或疤痕的皮肤上。

第 6 步:清洁
清洗您的双手。 用酒精擦拭片清洁注射部位。 注射前让皮肤干燥。
![]() 注射前请勿再次触摸注射部位或吹干注射部位。
注射前请勿再次触摸注射部位或吹干注射部位。

第 7 步:拉
手持注射器体中部,针头置于背离您的方向,并拔下针帽。

![]() 请勿将针帽重新盖上。
请勿将针帽重新盖上。 ![]() 请勿触碰针头。 取下针帽后立即注射药物。
请勿触碰针头。 取下针帽后立即注射药物。
第 8 步:捏 如图所示,在注射部位捏起一道皮肤。

第 9 步:插入
将针头以大约 45º 的角度完全插入捏起的皮肤处。

第 10 步:推
放松捏起的部位。 将柱塞杆缓慢且匀速地向下推,直至将注射器排空。 注意:您会感觉到一些阻力,此为正常现象。

第 11 步:释放和拔出 松开拇指并释放柱塞杆,直至针头防护装置包裹针头,然后从注射部位取下注射
器。
若出血,可以用棉球或纱布轻轻按住注射部位。。 ![]() 请勿将针帽重新盖上。
请勿将针帽重新盖上。 ![]() 注射后,请勿摩擦注射部位皮肤。
注射后,请勿摩擦注射部位皮肤。

第 12 步:处置
将注射器和针帽置于防刺穿容器中。 ![]() 请勿将针帽重新盖上。 始终将容器置于儿童不易接触的地方。
请勿将针帽重新盖上。 始终将容器置于儿童不易接触的地方。

Generic Name: dupilumab
Dosage Form: injection, solution
Medically reviewed by Drugs.com. Last updated on Jun 1, 2019.
OverviewSide EffectsDosageProfessionalInteractionsMoreDupixent is indicated for the following diseases:
Atopic DermatitisDupixent is indicated for the treatment of patients aged 12 years and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Dupixent can be used with or without topical corticosteroids.
AsthmaDupixent is indicated as an add-on maintenance treatment in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid dependent asthma.
Limitation of Use
Dupixent is not indicated for the relief of acute bronchospasm or status asthmaticus.
Chronic Rhinosinusitis with Nasal PolyposisDupixent is indicated as an add-on maintenance treatment in adult patients with inadequately controlled chronic rhinosinusitis with nasal polyposis (CRSwNP).
Dupixent Dosage and AdministrationDupixent is administered by subcutaneous injection.
Atopic DermatitisDosing in Adults
The recommended dose of Dupixent for adult patients is an initial dose of 600 mg (two 300 mg injections), followed by 300 mg given every other week.
Dosing in Adolescents
The recommended dose of Dupixent for patients 12 to 17 years of age is specified in Table 1.
| Body Weight | Initial Dose | Subsequent Doses (every other week) |
|---|---|---|
| less than 60 kg | 400 mg (two 200 mg injections) | 200 mg |
| 60 kg or more | 600 mg (two 300 mg injections) | 300 mg |
Concomitant Topical Therapies
Dupixent can be used with or without topical corticosteroids. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas.
AsthmaThe recommended dose of Dupixent for adults and adolescents (12 years of age and older) is:
an initial dose of 400 mg (two 200 mg injections) followed by 200 mg given every other week oran initial dose of 600 mg (two 300 mg injections) followed by 300 mg given every other weekfor patients with oral corticosteroids-dependent asthma, or with co-morbid moderate-to-severe atopic dermatitis for which Dupixent is indicated, start with an initial dose of 600 mg followed by 300 mg given every other weekThe recommended dose of Dupixent for adult patients is 300 mg given every other week.
Important Administration InstructionsDupixent is intended for use under the guidance of a healthcare provider. A patient may self-inject Dupixent after training in subcutaneous injection technique using the pre-filled syringe. Provide proper training to patients and/or caregivers on the preparation and administration of Dupixent prior to use according to the "Instructions for Use".
For atopic dermatitis and asthma patients taking an initial 600 mg dose, administer each of the two Dupixent 300 mg injections at different injection sites.
For asthma patients taking an initial 400 mg dose, administer each of the two Dupixent 200 mg injections at different injection sites.
Administer subcutaneous injection into the thigh or abdomen, except for the 2 inches (5 cm) around the navel. The upper arm can also be used if a caregiver administers the injection.
Rotate the injection site with each injection. DO NOT inject Dupixent into skin that is tender, damaged, bruised, or scarred.
If a dose is missed, instruct the patient to administer the injection within 7 days from the missed dose and then resume the patient's original schedule. If the missed dose is not administered within 7 days, instruct the patient to wait until the next dose on the original schedule.
The Dupixent "Instructions for Use" contains more detailed instructions on the preparation and administration of Dupixent [see Instructions for Use].
Preparation for Use of Dupixent Pre-filled Syringe with Needle ShieldBefore injection, remove Dupixent pre-filled syringe from the refrigerator and allow Dupixent to reach room temperature (45 minutes for the 300 mg/2 mL pre-filled syringe and 30 minutes for the 200 mg/1.14 mL pre-filled syringe) without removing the needle cap.
Inspect Dupixent visually for particulate matter and discoloration prior to administration. Dupixent is a clear to slightly opalescent, colorless to pale yellow solution. Do not use if the liquid contains visible particulate matter, is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow). Dupixent does not contain preservatives; therefore, discard any unused product remaining in the pre-filled syringe.
Dosage Forms and StrengthsDupixent is a clear to slightly opalescent, colorless to pale yellow solution available as:
Injection: 300 mg/2 mL in a single-dose pre-filled syringe with needle shieldInjection: 200 mg/1.14 mL in a single-dose pre-filled syringe with needle shieldContraindicationsDupixent is contraindicated in patients who have known hypersensitivity to dupilumab or any of its excipients [see Warnings and Precautions (5.1)].
Warnings and PrecautionsHypersensitivityHypersensitivity reactions, including generalized urticaria, rash, erythema nodosum and serum sickness or serum sickness-like reactions, were reported in less than 1% of subjects who received Dupixent in clinical trials. Two subjects in the atopic dermatitis development program experienced serum sickness or serum sickness-like reactions that were associated with high titers of antibodies to dupilumab. One subject in the asthma development program experienced anaphylaxis [see Adverse Reactions (6.2)]. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue Dupixent [see Adverse Reactions (6.1, 6.2)].
Conjunctivitis and KeratitisConjunctivitis and keratitis occurred more frequently in atopic dermatitis subjects who received Dupixent. Conjunctivitis was the most frequently reported eye disorder. Most subjects with conjunctivitis recovered or were recovering during the treatment period. Keratitis was reported in <1% of the Dupixent group (1 per 100 subject-years) and in 0% of the placebo group (0 per 100 subject-years) in the 16-week atopic dermatitis monotherapy trials. In the 52-week Dupixent + topical corticosteroids (TCS) atopic dermatitis trial, keratitis was reported in 4% of the Dupixent + TCS group (12 per 100 subject-years) and in 0% of the placebo + TCS group (0 per 100 subject-years). Most subjects with keratitis recovered or were recovering during the treatment period [see Adverse Reactions (6.1)].
Among asthma subjects, the frequencies of conjunctivitis and keratitis were similar between Dupixent and placebo [see Adverse Reactions (6.1)].
In subjects with CRSwNP, the frequency of conjunctivitis was 2% in the Dupixent group compared to 1% in the placebo group in the 24-week safety pool; these subjects recovered. There were no cases of keratitis reported in the CRSwNP development program [see Adverse Reactions (6.1)].
Advise patients to report new onset or worsening eye symptoms to their healthcare provider.
Eosinophilic ConditionsPatients being treated for asthma may present with serious systemic eosinophilia sometimes presenting with clinical features of eosinophilic pneumonia or vasculitis consistent with eosinophilic granulomatosis with polyangiitis, conditions which are often treated with systemic corticosteroid therapy. These events may be associated with the reduction of oral corticosteroid therapy. Physicians should be alert to vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients with eosinophilia. Cases of eosinophilic pneumonia were reported in adult patients who participated in the asthma development program and cases of vasculitis consistent with eosinophilic granulomatosis with polyangiitis have been reported with Dupixent in adult patients who participated in the asthma development program as well as in adult patients with co-morbid asthma in the CRSwNP development program. A causal association between Dupixent and these conditions has not been established.
Acute Asthma Symptoms or Deteriorating DiseaseDupixent should not be used to treat acute asthma symptoms or acute exacerbations. Do not use Dupixent to treat acute bronchospasm or status asthmaticus. Patients should seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with Dupixent.
Reduction of Corticosteroid DosageDo not discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of therapy with Dupixent. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Patients with Co-morbid AsthmaAdvise patients with atopic dermatitis or CRSwNP who have co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Parasitic (Helminth) InfectionsPatients with known helminth infections were excluded from participation in clinical studies. It is unknown if Dupixent will influence the immune response against helminth infections.
Treat patients with pre-existing helminth infections before initiating therapy with Dupixent. If patients become infected while receiving treatment with Dupixent and do not respond to anti-helminth treatment, discontinue treatment with Dupixent until the infection resolves.
Adverse ReactionsThe following adverse reactions are discussed in greater detail elsewhere in the labeling:
Hypersensitivity [see Warnings and Precautions (5.1)]Conjunctivitis and Keratitis [see Warnings and Precautions (5.2)]Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults with Atopic Dermatitis
Three randomized, double-blind, placebo-controlled, multicenter trials (Trials 1, 2, and 3) and one dose-ranging trial (Trial 4) evaluated the safety of Dupixent in subjects with moderate-to-severe atopic dermatitis. The safety population had a mean age of 38 years; 41% of subjects were female, 67% were white, 24% were Asian, and 6% were black; in terms of co-morbid conditions, 48% of the subjects had asthma, 49% had allergic rhinitis, 37% had food allergy, and 27% had allergic conjunctivitis. In these 4 trials, 1472 subjects were treated with subcutaneous injections of Dupixent, with or without concomitant topical corticosteroids (TCS).
A total of 739 subjects were treated with Dupixent for at least 1 year in the development program for moderate-to-severe atopic dermatitis.
Trials 1, 2, and 4 compared the safety of Dupixent monotherapy to placebo through Week 16. Trial 3 compared the safety of Dupixent + TCS to placebo + TCS through Week 52.
Weeks 0 to 16 (Trials 1 to 4):
In Dupixent monotherapy trials (Trials 1, 2, and 4) through Week 16, the proportion of subjects who discontinued treatment because of adverse events was 1.9% in both the Dupixent 300 mg Q2W and placebo groups.
Table 2 summarizes the adverse reactions that occurred at a rate of at least 1% in the Dupixent 300 mg Q2W monotherapy groups, and in the Dupixent + TCS group, all at a higher rate than in their respective comparator groups during the first 16 weeks of treatment.
| Adverse Reaction | Dupixent Monotherapy* | Dupixent + TCS† | ||
|---|---|---|---|---|
|
Dupixent 300 mg Q2W‡ |
Placebo |
Dupixent 300 mg Q2W‡ + TCS |
Placebo + TCS | |
|
N=529 n (%) |
N=517 n (%) |
N=110 n (%) |
N=315 n (%) |
|
| *Pooled analysis of Trials 1, 2, and 4.†Analysis of Trial 3 where subjects were on background TCS therapy.‡Dupixent 600 mg at Week 0, followed by 300 mg every two weeks.§Conjunctivitis cluster includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, giant papillary conjunctivitis, eye irritation, and eye inflammation.¶Keratitis cluster includes keratitis, ulcerative keratitis, allergic keratitis, atopic keratoconjunctivitis, and ophthalmic herpes simplex.#Other herpes simplex virus infection cluster includes herpes simplex, genital herpes, herpes simplex otitis externa, and herpes virus infection, but excludes eczema herpeticum. | ||||
| Injection site reaction | 51 (10) | 28 (5) | 11 (10) | 18 (6) |
| Conjunctivitis§ | 51 (10) | 12 (2) | 10 (9) | 15 (5) |
| Blepharitis | 2 (<1) | 1 (<1) | 5 (5) | 2 (1) |
| Oral herpes | 20 (4) | 8 (2) | 3 (3) | 5 (2) |
| Keratitis¶ | 1 (<1) | 0 | 4 (4) | 0 |
| Eye pruritus | 3 (1) | 1 (<1) | 2 (2) | 2 (1) |
| Other herpes simplex virus infection# | 10 (2) | 6 (1) | 1 (1) | 1 (<1) |
| Dry eye | 1 (<1) | 0 | 2 (2) | 1 (<1) |
Safety through Week 52 (Trial 3):
In the Dupixent with concomitant TCS trial (Trial 3) through Week 52, the proportion of subjects who discontinued treatment because of adverse events was 1.8% in Dupixent 300 mg Q2W + TCS group and 7.6% in the placebo + TCS group. Two subjects discontinued Dupixent because of adverse reactions: atopic dermatitis (1 subject) and exfoliative dermatitis (1 subject).
The safety profile of Dupixent + TCS through Week 52 was generally consistent with the safety profile observed at Week 16.
Adolescents with Atopic Dermatitis
The safety of Dupixent was assessed in a trial of 250 subjects 12 to 17 years of age with moderate-to-severe atopic dermatitis (Trial 6). The safety profile of Dupixent in these subjects through Week 16 was similar to the safety profile from studies in adults with atopic dermatitis.
The long-term safety of Dupixent was assessed in an open-label extension study in subjects 12 to 17 years of age with moderate-to-severe atopic dermatitis (Trial 7). The safety profile of Dupixent in subjects followed through Week 52 was similar to the safety profile observed at Week 16 in Trial 6. The long-term safety profile of Dupixent observed in adolescents was consistent with that seen in adults with atopic dermatitis.
Asthma
A total of 2888 adult and adolescent subjects with moderate-to-severe asthma (AS) were evaluated in 3 randomized, placebo-controlled, multicenter trials of 24 to 52 weeks duration (AS Trials 1, 2, and 3). Of these, 2678 had a history of 1 or more severe exacerbations in the year prior to enrollment despite regular use of medium to high-dose inhaled corticosteroids plus an additional controller(s) (AS Trials 1 and 2). A total of 210 subjects with oral corticosteroid-dependent asthma receiving high-dose inhaled corticosteroids plus up to two additional controllers were enrolled (AS Trial 3). The safety population (AS Trials 1 and 2) was 12-87 years of age, of which 63% were female, and 82% were white. Dupixent 200 mg or 300 mg was administered subcutaneously Q2W, following an initial dose of 400 mg or 600 mg, respectively.
In AS Trials 1 and 2, the proportion of subjects who discontinued treatment due to adverse events was 4% of the placebo group, 3% of the Dupixent 200 mg Q2W group, and 6% of the Dupixent 300 mg Q2W group.
Table 3 summarizes the adverse reactions that occurred at a rate of at least 1% in subjects treated with Dupixent and at a higher rate than in their respective comparator groups in Asthma Trials 1 and 2.
| Adverse Reaction | AS Trials 1 and 2 | ||
|---|---|---|---|
|
Dupixent 200 mg Q2W |
Dupixent 300 mg Q2W |
Placebo | |
|
N=779 n (%) |
N=788 n (%) |
N=792 n (%) |
|
| *Injection site reactions cluster includes erythema, edema, pruritus, pain, and inflammation.†Eosinophilia = blood eosinophils ≥3,000 cells/mcL, or deemed by the investigator to be an adverse event. None met the criteria for serious eosinophilic conditions [see Warnings and Precautions (5.3)]. | |||
| Injection site reactions* | 111 (14%) | 144 (18%) | 50 (6%) |
| Oropharyngeal pain | 13 (2%) | 19 (2%) | 7 (1%) |
| Eosinophilia† | 17 (2%) | 16 (2%) | 2 (<1%) |
Injection site reactions were most common with the loading (initial) dose.
The safety profile of Dupixent through Week 52 was generally consistent with the safety profile observed at Week 24.
Chronic Rhinosinusitis with Nasal Polyposis
A total of 722 adult subjects with chronic rhinosinusitis with nasal polyposis (CRSwNP) were evaluated in 2 randomized, placebo-controlled, multicenter trials of 24 to 52 weeks duration (CSNP Trials 1 and 2). The safety pool consisted of data from the first 24 weeks of treatment from both studies.
In the safety pool, the proportion of subjects who discontinued treatment due to adverse events was 5% of the placebo group and 2% of the Dupixent 300 mg Q2W group.
Table 4 summarizes the adverse reactions that occurred at a rate of at least 1% in subjects treated with Dupixent and at a higher rate than in their respective comparator group in CSNP Trials 1 and 2.
| Adverse Reaction | CSNP Trials 1 and 2 | |
|---|---|---|
|
Dupixent 300 mg Q2W |
Placebo | |
|
N=440 n (%) |
N=282 n (%) |
|
| *Injection site reactions cluster includes injection site reaction, pain, bruising and swelling.†Conjunctivitis cluster includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, giant papillary conjunctivitis, eye irritation, and eye inflammation. | ||
| Injection site reactions* | 28 (6%) | 12 (4%) |
| Conjunctivitis† | 7 (2%) | 2 (1%) |
| Arthralgia | 14 (3%) | 5 (2%) |
| Gastritis | 7 (2%) | 2 (1%) |
| Insomnia | 6 (1%) | 0 (<1%) |
| Eosinophilia | 5 (1%) | 1 (<1%) |
| Toothache | 5 (1%) | 1 (<1%) |
The safety profile of Dupixent through Week 52 was generally consistent with the safety profile observed at Week 24.
Specific Adverse Reactions
Conjunctivitis
During the 52-week treatment period of concomitant therapy atopic dermatitis trial (Trial 3), conjunctivitis was reported in 16% of the Dupixent 300 mg Q2W + TCS group (20 per 100 subject-years) and in 9% of the placebo + TCS group (10 per 100 subject-years). Among asthma subjects, the frequency of conjunctivitis was similar between Dupixent and placebo. In the 52-week CRSwNP study (CSNP Trial 2), the frequency of conjunctivitis was 3% in the Dupixent subjects and 1% in the placebo subjects; all of these subjects recovered. [see Warnings and Precautions (5.2)].
Eczema Herpeticum and Herpes Zoster
The rate of eczema herpeticum was similar in the placebo and Dupixent groups in the atopic dermatitis trials.
Herpes zoster was reported in <0.1% of the Dupixent groups (<1 per 100 subject-years) and in <1% of the placebo group (1 per 100 subject-years) in the 16-week atopic dermatitis monotherapy trials. In the 52-week Dupixent + TCS atopic dermatitis trial, herpes zoster was reported in 1% of the Dupixent + TCS group (1 per 100 subject-years) and 2% of the placebo + TCS group (2 per 100 subject-years). Among asthma subjects the frequency of herpes zoster was similar between Dupixent and placebo. Among CRSwNP subjects there were no reported cases of herpes zoster or eczema herpeticum.
Hypersensitivity Reactions
Hypersensitivity reactions were reported in <1% of Dupixent-treated subjects. These included serum sickness reaction, serum sickness-like reaction, generalized urticaria, rash, erythema nodosum, and anaphylaxis [see Contraindications (4), Warnings and Precautions (5.1), and Adverse Reactions (6.2)].
Eosinophils
Dupixent-treated subjects had a greater initial increase from baseline in blood eosinophil count compared to subjects treated with placebo. In subjects with atopic dermatitis, the mean and median increases in blood eosinophils from baseline to Week 4 were 100 and 0 cells/mcL respectively. In subjects with asthma, the mean and median increases in blood eosinophils from baseline to Week 4 were 130 and 10 cells/mcL respectively. In subjects with CRSwNP, the mean and median increases in blood eosinophils from baseline to Week 16 were 150 and 50 cells/mcL, respectively.
Across all indications, the incidence of treatment-emergent eosinophilia (≥500 cells/mcL) was similar in Dupixent and placebo groups. Treatment-emergent eosinophilia (≥5,000 cells/mcL) was reported in <2% of Dupixent-treated patients and <0.5% in placebo-treated patients. Blood eosinophil counts declined to near baseline levels during study treatment [see Warnings and Precautions (5.3)].
Cardiovascular
In the 1-year placebo controlled trial in subjects with asthma (AS Trial 2), cardiovascular thromboembolic events (cardiovascular deaths, non-fatal myocardial infarctions, and non-fatal strokes) were reported in 1 (0.2%) of the Dupixent 200 mg Q2W group, 4 (0.6%) of the Dupixent 300 mg Q2W group, and 2 (0.3%) of the placebo group.
In the 1-year placebo controlled trial in subjects with atopic dermatitis (Trial 3), cardiovascular thromboembolic events (cardiovascular deaths, non-fatal myocardial infarctions, and non-fatal strokes) were reported in 1 (0.9%) of the Dupixent + TCS 300 mg Q2W group, 0 (0.0%) of the Dupixent + TCS 300 mg QW group, and 1 (0.3%) of the placebo + TCS group.
In the 24-week placebo controlled trial in subjects with CRSwNP (CSNP Trial 1), cardiovascular thromboembolic events (cardiovascular deaths, non-fatal myocardial infarctions, and non-fatal strokes) were reported in 1 (0.7%) of the Dupixent group and 0 (0.0%) of the placebo group. In the 1-year placebo controlled trial in subjects with CRSwNP (CSNP Trial 2), there were no cases of cardiovascular thromboembolic events (cardiovascular deaths, non-fatal myocardial infarctions, and non-fatal strokes) reported in any treatment arm.
ImmunogenicityAs with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to dupilumab in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Approximately 5% of subjects with atopic dermatitis, asthma, or CRSwNP who received Dupixent 300 mg Q2W for 52 weeks developed antibodies to dupilumab; approximately 2% exhibited persistent ADA responses, and approximately 2% had neutralizing antibodies.
Approximately 9% of subjects with asthma who received Dupixent 200 mg Q2W for 52 weeks developed antibodies to dupilumab; approximately 4% exhibited persistent ADA responses, and approximately 4% had neutralizing antibodies.
Approximately 4% of subjects in the placebo groups in the 52-week studies were positive for antibodies to Dupixent; approximately 2% exhibited persistent ADA responses, and approximately 1% had neutralizing antibodies.
Approximately 16% of adolescent subjects with atopic dermatitis who received Dupixent 300 mg or 200 mg Q2W for 16 weeks developed antibodies to dupilumab; approximately 3% exhibited persistent ADA responses, and approximately 5% had neutralizing antibodies.
Approximately 4% of adolescent subjects with atopic dermatitis in the placebo group were positive for antibodies to Dupixent; approximately 1% exhibited persistent ADA responses, and approximately 1% had neutralizing antibodies.
The antibody titers detected in both Dupixent and placebo subjects were mostly low. In subjects who received Dupixent, development of high titer antibodies to dupilumab was associated with lower serum dupilumab concentrations [see Clinical Pharmacology (12.3)].
Two subjects who experienced high titer antibody responses developed serum sickness or serum sickness-like reactions during Dupixent therapy [see Warnings and Precautions (5.1)].
Drug InteractionsLive VaccinesAvoid use of live vaccines in patients treated with Dupixent.
Non-Live VaccinesImmune responses to vaccination were assessed in a study in which subjects with atopic dermatitis were treated once weekly for 16 weeks with 300 mg of dupilumab (twice the recommended dosing frequency). After 12 weeks of Dupixent administration, subjects were vaccinated with a Tdap vaccine (Adacel®) and a meningococcal polysaccharide vaccine (Menomune®). Antibody responses to tetanus toxoid and serogroup C meningococcal polysaccharide were assessed 4 weeks later. Antibody responses to both tetanus vaccine and meningococcal polysaccharide vaccine were similar in dupilumab-treated and placebo-treated subjects. Immune responses to the other active components of the Adacel and Menomune vaccines were not assessed.
USE IN SPECIFIC POPULATIONSPregnancyPregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Dupixent during pregnancy.
Please contact 1-877-311-8972 or go to https://mothertobaby.org/ongoing-study/Dupixent/ to enroll in or to obtain information about the registry.
Risk Summary
Available data from case reports and case series with Dupixent use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Human IgG antibodies are known to cross the placental barrier; therefore, Dupixent may be transmitted from the mother to the developing fetus. There are adverse effects on maternal and fetal outcomes associated with asthma in pregnancy (see Clinical Considerations). In an enhanced pre- and post-natal developmental study, no adverse developmental effects were observed in offspring born to pregnant monkeys after subcutaneous administration of a homologous antibody against interleukin-4-receptor alpha (IL-4Rα) during organogenesis through parturition at doses up to 10-times the maximum recommended human dose (MRHD) (see Data). The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-fetal Risk
In women with poorly or moderately controlled asthma, evidence demonstrates that there is an increased risk of preeclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. The level of asthma control should be closely monitored in pregnant women and treatment adjusted as necessary to maintain optimal control.
Data
Animal Data
In an enhanced pre- and post-natal development toxicity study, pregnant cynomolgus monkeys were administered weekly subcutaneous doses of homologous antibody against IL-4Rα up to 10 times the MRHD (on a mg/kg basis of 100 mg/kg/week) from the beginning of organogenesis to parturition. No treatment-related adverse effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in the infants from birth through 6 months of age.
LactationRisk Summary
There are no data on the presence of dupilumab in human milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure to dupilumab on the breastfed infant are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Dupixent and any potential adverse effects on the breastfed child from Dupixent or from the underlying maternal condition.
Pediatric UseAtopic Dermatitis
The safety and efficacy of Dupixent have been established in pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis. A total of 251 adolescents ages 12 to 17 years old with moderate-to-severe atopic dermatitis were enrolled in Trial 6. The safety and efficacy were generally consistent between adolescents and adults [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. Safety and efficacy in pediatric patients (<12 years of age) with atopic dermatitis have not been established.
Asthma
A total of 107 adolescents aged 12 to 17 years with moderate-to-severe asthma were enrolled in AS Trial 2 and received either 200 mg (N=21) or 300 mg (N=18) Dupixent (or matching placebo either 200 mg [N=34] or 300 mg [N=34]) Q2W. Asthma exacerbations and lung function were assessed in both adolescents and adults. For both the 200 mg and 300 mg Q2W doses, improvements in FEV1 (LS mean change from baseline at Week 12) were observed (0.36 L and 0.27 L, respectively). For the 200 mg Q2W dose, subjects had a reduction in the rate of severe exacerbations that was consistent with adults. Safety and efficacy in pediatric patients (<12 years of age) with asthma have not been established. Dupilumab exposure was higher in adolescent patients than that in adults at the respective dose level which was mainly accounted for by difference in body weight [see Clinical Pharmacology (12.3)].
The adverse event profile in adolescents was generally similar to the adults [see Adverse Reactions (6.1)].
CRSwNP
CRSwNP does not normally occur in children. Safety and efficacy in pediatric patients (<18 years of age) with CRSwNP have not been established.
Geriatric UseOf the 1472 subjects with atopic dermatitis exposed to Dupixent in a dose-ranging study and placebo-controlled trials, 67 subjects were 65 years or older. Although no differences in safety or efficacy were observed between older and younger subjects, the number of subjects aged 65 and over is not sufficient to determine whether they respond differently from younger subjects [see Clinical Pharmacology (12.3)].
Of the 1977 subjects with asthma exposed to Dupixent, a total of 240 subjects were 65 years or older. Efficacy and safety in this age group was similar to the overall study population.
Of the 440 subjects with CRSwNP exposed to Dupixent, a total of 79 subjects were 65 years or older. Efficacy and safety in this age group were similar to the overall study population.
There is no specific treatment for Dupixent overdose. In the event of overdosage, monitor the patient for any signs or symptoms of adverse reactions and institute appropriate symptomatic treatment immediately.
Dupixent DescriptionDupilumab, an interleukin-4 receptor alpha antagonist, is a human monoclonal antibody of the IgG4 subclass that binds to the IL-4Rα subunit and inhibits IL-4 and IL-13 signaling. Dupilumab has an approximate molecular weight of 147 kDa.
Dupilumab is produced by recombinant DNA technology in Chinese Hamster Ovary cell suspension culture.
Dupixent (dupilumab) Injection is supplied as a sterile, preservative-free, clear to slightly opalescent, colorless to pale yellow solution for subcutaneous injection. Dupixent is provided as a single-dose pre-filled syringe with needle shield in a siliconized Type-1 clear glass syringe. The needle cap is not made with natural rubber latex.
Each 300 mg pre-filled syringe delivers 300 mg dupilumab in 2 mL which also contains L-arginine hydrochloride (10.5 mg), L-histidine (6.2 mg), polysorbate 80 (4 mg), sodium acetate (2 mg), sucrose (100 mg), and water for injection, pH 5.9.
Each 200 mg pre-filled syringe delivers 200 mg dupilumab in 1.14 mL which also contains L-arginine hydrochloride (12 mg), L-histidine (3.5 mg), polysorbate 80 (2.3 mg), sodium acetate (1.2 mg), sucrose (57 mg), and water for injection, pH 5.9.
Dupixent - Clinical PharmacologyMechanism of ActionDupilumab is a human monoclonal IgG4 antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling by specifically binding to the IL-4Rα subunit shared by the IL-4 and IL-13 receptor complexes. Dupilumab inhibits IL-4 signaling via the Type I receptor and both IL-4 and IL-13 signaling through the Type II receptor.
Inflammation is an important component in the pathogenesis of asthma, atopic dermatitis, and CRSwNP. Multiple cell types that express IL-4Rα (e.g., mast cells, eosinophils, macrophages, lymphocytes, epithelial cells, goblet cells) and inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines, chemokines) are involved in inflammation. Blocking IL-4Rα with dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE; however, the mechanism of dupilumab action in asthma has not been definitively established.
PharmacodynamicsConsistent with inhibition of IL-4 and IL-13 signaling, dupilumab treatment decreased certain biomarkers. In asthma subjects, fractional exhaled nitric oxide (FeNO) and circulating concentrations of eotaxin-3, total IgE, allergen specific IgE, TARC, and periostin were decreased relative to placebo. These reductions in biomarkers were comparable for the 300 mg Q2W and 200 mg Q2W regimens. These markers were near maximal suppression after 2 weeks of treatment, except for IgE which declined more slowly. These effects were sustained throughout treatment. The median percent reduction from baseline in total IgE concentrations with dupilumab treatments was 52% at Week 24 (AS Trial 1) and 70% at Week 52 (AS Trial 2). For FeNO, the mean percent reduction from baseline at Week 2 was 35% and 24% in AS Trials 1 and 2 respectively, and in the overall safety population, the mean FeNO level decreased to 20 ppb.
PharmacokineticsThe pharmacokinetics of dupilumab is similar in subjects with atopic dermatitis, asthma, and CRSwNP.
Absorption
Following an initial subcutaneous (SC) dose of 600 mg, 400 mg, or 300 mg, dupilumab reached peak mean ± SD concentrations (Cmax) of 70.1±24.1 mcg/mL, 41.8±12.4 mcg/mL, or 30.5±9.39 mcg/mL respectively, by approximately 1 week post dose. Steady-state concentrations were achieved by Week 16 following the administration of 600 mg starting dose and 300 mg dose either weekly (twice the recommended dosing frequency) or Q2W, or 400 mg starting dose and 200 mg dose Q2W, or 300 mg Q2W without a loading dose. Across clinical trials, the mean ± SD steady-state trough concentrations ranged from 60.3±35.1 mcg/mL to 80.2±35.3 mcg/mL for 300 mg administered Q2W, from 173±75.9 mcg/mL to 193±77.0 mcg/mL for 300 mg administered weekly, and from 29.2±18.7 to 36.5±22.2 mg/L for 200 mg administered Q2W.
The bioavailability of dupilumab following a SC dose is similar between AD, asthma, and CRSwNP patients, ranging between 61% and 64%.
Distribution
The estimated total volume of distribution was approximately 4.8±1.3 L.
Elimination
The metabolic pathway of dupilumab has not been characterized. As a human monoclonal IgG4 antibody, dupilumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG. After the last steady-state dose of 300 mg Q2W, 300 mg QW, or 200 mg Q2W dupilumab, the median times to non-detectable concentration (<78 ng/mL) are 10-12, 13, and 9 weeks, respectively.
Dose Linearity
Dupilumab exhibited nonlinear target-mediated pharmacokinetics with exposures increasing in a greater than dose-proportional manner. The systemic exposure increased by 30-fold when the dose increased 8-fold following a single dose of dupilumab from 75 mg to 600 mg (i.e., 0.25-times to 2-times the recommended dose).
Weight
Dupilumab trough concentrations were lower in subjects with higher body weight.
Age
Based on population pharmacokinetic analysis, age did not affect dupilumab clearance.
Immunogenicity
Development of antibodies to dupilumab was associated with lower serum dupilumab concentrations. A few subjects who had high antibody titers also had no detectable serum dupilumab concentrations.
Specific Populations
Geriatric Patients
In subjects who are 65 years and older, the mean ± SD steady-state trough concentrations of dupilumab were 69.4±31.4 mcg/mL and 166±62.3 mcg/mL, respectively, for 300 mg administered Q2W and weekly, and 39.7±21.7 mcg/mL for 200 mg administered Q2W.
Pediatric Patients
Atopic Dermatitis
For adolescents 12 to 17 years of age with atopic dermatitis receiving every other week dosing (Q2W) with either 200 mg (<60 kg) or 300 mg (≥60 kg), the mean ± SD steady-state trough concentration of dupilumab was 54.5±27.0 mcg/mL.
Asthma
A total of 107 adolescents aged 12 to 17 years with asthma were enrolled in AS Trial 2. The mean ± SD steady-state trough concentrations of dupilumab were 107±51.6 mcg/mL and 46.7±26.9 mcg/mL, respectively, for 300 mg or 200 mg administered Q2W.
Renal or Hepatic Impairment
No formal trial of the effect of hepatic or renal impairment on the pharmacokinetics of dupilumab was conducted.
Drug Interaction Studies
An effect of dupilumab on the PK of co-administered medications is not expected. Based on the population analysis, commonly co-administered medications had no effect on Dupixent pharmacokinetics in patients with moderate-to-severe asthma.
Cytochrome P450 Substrates
The effects of dupilumab on the pharmacokinetics of midazolam (metabolized by CYP3A4), warfarin (metabolized by CYP2C9), omeprazole (metabolized by CYP2C19), metoprolol (metabolized by CYP2D6), and caffeine (metabolized by CYP1A2) were evaluated in a study with 12-13 evaluable subjects with atopic dermatitis (a SC loading dose of 600 mg followed by 300 mg SC weekly for six weeks). No clinically significant changes in AUC were observed. The largest effect was observed for metoprolol (CYP2D6) with an increase in AUC of 29%.
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of dupilumab.
No effects on fertility parameters such as reproductive organs, menstrual cycle length, or sperm analysis were observed in sexually mature mice that were subcutaneously administered a homologous antibody against IL-4Rα at doses up to 200 mg/kg/week.
Clinical StudiesAtopic DermatitisAdults with Atopic Dermatitis
Three randomized, double-blind, placebo-controlled trials (Trials 1, 2, and 3; NCT02277743, 02277769, and 02260986 respectively) enrolled a total of 2119 subjects 18 years of age and older with moderate-to-severe atopic dermatitis (AD) not adequately controlled by topical medication(s). Disease severity was defined by an Investigator's Global Assessment (IGA) score ≥3 in the overall assessment of AD lesions on a severity scale of 0 to 4, an Eczema Area and Severity Index (EASI) score ≥16 on a scale of 0 to 72, and a minimum body surface area involvement of ≥10%. At baseline, 59% of subjects were male, 67% were white, 52% of subjects had a baseline IGA score of 3 (moderate AD), and 48% of subjects had a baseline IGA of 4 (severe AD). The baseline mean EASI score was 33 and the baseline weekly averaged Peak Pruritus Numeric Rating Scale (NRS) was 7 on a scale of 0-10.
In all three trials, subjects in the Dupixent group received subcutaneous injections of Dupixent 600 mg at Week 0, followed by 300 mg every other week (Q2W). In the monotherapy trials (Trials 1 and 2), subjects received Dupixent or placebo for 16 weeks.
In the concomitant therapy trial (Trial 3), subjects received Dupixent or placebo with concomitant topical corticosteroids (TCS) and as needed topical calcineurin inhibitors for problem areas only, such as the face, neck, intertriginous and genital areas for 52 weeks.
All three trials assessed the primary endpoint, the change from baseline to Week 16 in the proportion of subjects with an IGA 0 (clear) or 1 (almost clear) and at least a 2-point improvement. Other endpoints included the proportion of subjects with EASI-75 (improvement of at least 75% in EASI score from baseline), and reduction in itch as defined by at least a 4-point improvement in the Peak Pruritus NRS from baseline to Week 16.
Clinical Response at Week 16 (Trials 1, 2, and 3)
The results of the Dupixent monotherapy trials (Trials 1 and 2) and the Dupixent with concomitant TCS trial (Trial 3) are presented in Table 5.
| Trial 1 | Trial 2 | Trial 3 | ||||
|---|---|---|---|---|---|---|
|
Dupixent 300 mg Q2W |
Placebo |
Dupixent 300 mg Q2W |
Placebo |
Dupixent 300 mg Q2W + TCS |
Placebo + TCS | |
| *Full Analysis Set (FAS) includes all subjects randomized.†Responder was defined as a subject with an IGA 0 or 1 ("clear" or "almost clear") and a reduction of ≥2 points on a 0-4 IGA scale.‡Subjects who received rescue treatment or with missing data were considered as non-responders. | ||||||
| Number of subjects randomized (FAS)* | 224 | 224 | 233 | 236 | 106 | 315 |
| IGA 0 or 1†,‡ | 38% | 10% | 36% | 9% | 39% | 12% |
| EASI-75‡ | 51% | 15% | 44% | 12% | 69% | 23% |
| EASI-90‡ | 36% | 8% | 30% | 7% | 40% | 11% |
| Number of subjects with baseline Peak Pruritus NRS score ≥4 | 213 | 212 | 225 | 221 | 102 | 299 |
|
Peak Pruritus NRS (≥4-point improvement)‡ |
41% | 12% | 36% | 10% | 59% | 20% |
| *In the primary analyses of the efficacy endpoints, subjects who received rescue treatment or with missing data were considered non-responders.†Full Analysis Set (FAS) includes all subjects randomized. | |||
| Figure 1: Proportion of Subjects with ≥4-point Improvement on the Peak Pruritus NRS in Trial 1* and Trial 2* Studies (FAS)† | |||
| Trial 1 | Trial 2 | ||
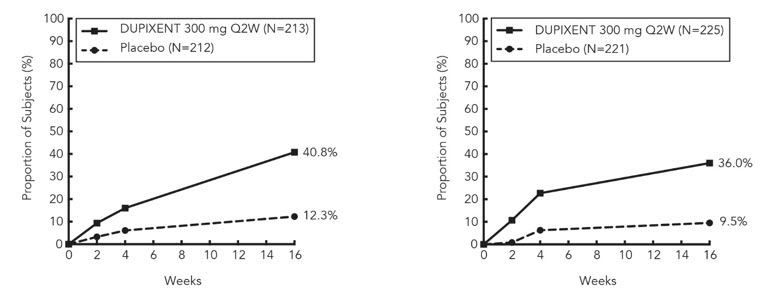
|
|
||
In Trial 3, of the 421 subjects, 353 had been on study for 52 weeks at the time of data analysis. Of these 353 subjects, responders at Week 52 represent a mixture of subjects who maintained their efficacy from Week 16 (e.g., 53% of Dupixent IGA 0 or 1 responders at Week 16 remained responders at Week 52) and subjects who were non-responders at Week 16 who later responded to treatment (e.g., 24% of Dupixent IGA 0 or 1 non-responders at Week 16 became responders at Week 52). Results of supportive analyses of the 353 subjects in the Dupixent with concomitant TCS trial (Trial 3) are presented in Table 6.
|
Dupixent 300 mg Q2W + TCS |
Placebo + TCS | |
|---|---|---|
| *In Trial 3, of the 421 randomized and treated subjects, 68 subjects (16%) had not been on study for 52 weeks at the time of data analysis.†Responder was defined as a subject with an IGA 0 or 1 ("clear" or "almost clear") and a reduction of ≥2 points on a 0-4 IGA scale.‡Subjects who received rescue treatment or with missing data were considered as non-responders. | ||
| Number of Subjects* | 89 | 264 |
| Responder†,‡ at Week 16 and 52 | 22% | 7% |
| Responder at Week 16 but Non-responder at Week 52 | 20% | 7% |
| Non-responder at Week 16 and Responder at Week 52 | 13% | 6% |
| Non-responder at Week 16 and 52 | 44% | 80% |
| Overall Responder†,‡ Rate at Week 52 | 36% | 13% |
Treatment effects in subgroups (weight, age, gender, race, and prior treatment, including immunosuppressants) in Trials 1, 2, and 3 were generally consistent with the results in the overall study population.
In Trials 1, 2, and 3, a third randomized treatment arm of Dupixent 300 mg QW did not demonstrate additional treatment benefit over Dupixent 300 mg Q2W.
Subjects in Trials 1 and 2 who had an IGA 0 or 1 with a reduction of ≥2 points were re-randomized into Trial 5. Trial 5 evaluated multiple Dupixent monotherapy dose regimens for maintaining treatment response. The study included subjects randomized to continue with Dupixent 300 mg Q2W (62 subjects) or switch to placebo (31 subjects) for 36 weeks. IGA 0 or 1 responses at Week 36 were as follows: 33 (53%) in the Q2W group and 3 (10%) in the placebo group.
Adolescents with Atopic Dermatitis
The efficacy and safety of Dupixent monotherapy in adolescent subjects was evaluated in a multicenter, randomized, double-blind, placebo-controlled trial (Trial 6; NCT03054428) in 251 adolescent subjects 12 to 17 years of age, with moderate-to-severe AD defined by an IGA score ≥3 (scale of 0 to 4), an EASI score ≥16 (scale of 0 to 72), and a minimum BSA involvement of ≥10%. Eligible subjects enrolled into this trial had previous inadequate response to topical medication.
Subjects in the Dupixent group with baseline weight of <60 kg received an initial dose of 400 mg at Week 0, followed by 200 mg Q2W for 16 weeks. Subjects with baseline weight of ≥60 kg received an initial dose of 600 mg at Week 0, followed by 300 mg Q2W for 16 weeks. Subjects were permitted to receive rescue treatment at the discretion of the investigator. Subjects who received rescue treatment were considered non-responders.
In Trial 6, the mean age was 14.5 years, the median weight was 59.4 kg, 41% of subjects were female, 63% were White, 15% were Asian, and 12% were Black. At baseline 46% of subjects had an IGA score of 3 (moderate AD), 54% had an IGA score of 4 (severe AD), the mean BSA involvement was 57%, and 42% had received prior systemic immunosuppressants. Also, at baseline the mean EASI score was 36, and the weekly averaged Peak Pruritus NRS was 8 on a scale of 0-10. Overall, 92% of subjects had at least one co-morbid allergic condition; 66% had allergic rhinitis, 54% had asthma, and 61% had food allergies.
The primary endpoint was the proportion of subjects with an IGA 0 (clear) or 1 (almost clear) and at least a 2-point improvement from baseline to Week 16. Other evaluated outcomes included the proportion of subjects with EASI-75 or EASI-90 (improvement of at least 75% or 90% in EASI from baseline, respectively), and reduction in itch as measured by the Peak Pruritus NRS (≥4-point improvement).
The efficacy results at Week 16 for Trial 6 are presented in Table 7.
|
Dupixent† 200 mg (<60 kg) or 300 mg (≥60 kg) Q2W N=82* |
Placebo N=85* |
|
|---|---|---|
| *Full Analysis Set (FAS) includes all subjects randomized.†At Week 0, subjects received 400 mg (baseline weight <60 kg) or 600 mg (baseline weight ≥60 kg) of Dupixent.‡Responder was defined as a subject with an IGA 0 or 1 ("clear" or "almost clear") and a reduction of ≥2 points on a 0-4 IGA scale.§Subjects who received rescue treatment or with missing data were considered as non-responders (59% and 21% in the placebo and Dupixent arms, respectively). | ||
| IGA 0 or 1‡,§ | 24% | 2% |
| EASI-75§ | 42% | 8% |
| EASI-90§ | 23% | 2% |
| Peak Pruritus NRS (≥4-point improvement)§ | 37% | 5% |
A greater proportion of subjects randomized to Dupixent achieved an improvement in the Peak Pruritus NRS compared to placebo (defined as ≥4-point improvement at Week 4). See Figure 2.
| *In the primary analyses of the efficacy endpoints, subjects who received rescue treatment or with missing data were considered non-responders.†Full Analysis Set (FAS) includes all subjects randomized. |
| Figure 2: Proportion of Adolescent Subjects with ≥4-point Improvement on the Peak Pruritus NRS in Trial 6* (FAS)† |
|
The asthma development program included three randomized, double-blind, placebo-controlled, parallel-group, multi-center trials (AS Trials 1, 2, and 3) of 24 to 52 weeks in treatment duration which enrolled a total of 2888 subjects (12 years of age and older). Subjects enrolled in AS Trials 1 and 2 were required to have a history of 1 or more asthma exacerbations that required treatment with systemic corticosteroids or emergency department visit or hospitalization for the treatment of asthma in the year prior to trial entry. Subjects enrolled in AS Trial 3 required dependence on daily oral corticosteroids in addition to regular use of high-dose inhaled corticosteroids plus an additional controller(s). In all 3 trials, subjects were enrolled without requiring a minimum baseline blood eosinophil count. In AS Trials 2 and 3 subjects with screening blood eosinophil level of >1500 cells/mcL (<1.3%) were excluded. Dupixent was administered as add-on to background asthma treatment. Subjects continued background asthma therapy throughout the duration of the studies, except in AS Trial 3 in which OCS dose was tapered as described below.
AS Trial 1
AS Trial 1 was a 24-week dose-ranging study which included 776 subjects (18 years of age and older). Dupixent compared with placebo was evaluated in adult subjects with moderate-to-severe asthma on a medium or high-dose inhaled corticosteroid and a long acting beta agonist. Subjects were randomized to receive either 200 mg (N=150) or 300 mg (N=157) Dupixent every other week (Q2W) or 200 mg (N=154) or 300 mg (N=157) Dupixent every 4 weeks following an initial dose of 400 mg, 600 mg or placebo (N=158), respectively. The primary endpoint was mean change from baseline to Week 12 in FEV1 (L) in subjects with baseline blood eosinophils ≥300 cells/mcL. Other endpoints included percent change from baseline in FEV1 and annualized rate of severe asthma exacerbation events during the 24-week placebo controlled treatment period. Results were evaluated in the overall population and subgroups based on baseline blood eosinophil count (≥300 cells/mcL and <300 cells/mcL). Additional secondary endpoints included responder rates in the patient reported Asthma Control Questionnaire (ACQ-5) and Asthma Quality of Life Questionnaire, Standardized Version (AQLQ(S)) scores.
AS Trial 2
AS Trial 2 was a 52-week study which included 1902 subjects (12 years of age and older). Dupixent compared with placebo was evaluated in 107 adolescent and 1795 adult subjects with moderate-to-severe asthma on a medium or high-dose inhaled corticosteroid (ICS) and a minimum of one and up to two additional controller medications. Subjects were randomized to receive either 200 mg (N=631) or 300 mg (N=633) Dupixent Q2W (or matching placebo for either 200 mg [N=317] or 300 mg [N=321] Q2W) following an initial dose of 400 mg, 600 mg or placebo respectively. The primary endpoints were the annualized rate of severe exacerbation events during the 52-week placebo controlled period and change from baseline in pre-bronchodilator FEV1 at Week 12 in the overall population (unrestricted by minimum baseline blood eosinophils count). Additional secondary endpoints included annualized severe exacerbation rates and FEV1 in patients with different baseline levels of blood eosinophils as well as responder rates in the ACQ-5 and AQLQ(S) scores.
AS Trial 3
AS Trial 3 was a 24-week oral corticosteroid-reduction study in 210 subjects with asthma who required daily oral corticosteroids in addition to regular use of high dose inhaled corticosteroids plus an additional controller. After optimizing the OCS dose during the screening period, subjects received 300 mg Dupixent (N=103) or placebo (N=107) once Q2W for 24 weeks following an initial dose of 600 mg or placebo. Subjects continued to receive their existing asthma medicine during the study; however their OCS dose was reduced every 4 weeks during the OCS reduction phase (Week 4-20), as long as asthma control was maintained. The primary endpoint was the percent reduction of oral corticosteroid dose at Weeks 20 to 24 compared with the baseline dose, while maintaining asthma control in the overall population (unrestricted by minimum baseline blood eosinophils count). Additional secondary endpoints included the annualized rate of severe exacerbation events during treatment period and responder rate in the ACQ-5 and AQLQ(S) scores.
The demographics and baseline characteristics of these 3 trials are provided in Table 8 below.
| Parameter |
Trial 1 (N=776) |
Trial 2 (N=1902) |
Trial 3 (N=210) |
|---|---|---|---|
| ICS = inhaled corticosteroid; FEV1 = Forced expiratory volume in 1 second; AD = atopic dermatitis; NP = nasal polyposis; AR = allergic rhinitis; FeNO = fraction of exhaled nitric oxide | |||
| Mean age (years) (SD) | 49 (13) | 48 (15) | 51 (13) |
| % Female | 63 | 63 | 61 |
| % White | 78 | 83 | 94 |
| Duration of Asthma (years), mean (± SD) | 22 (15) | 21 (15) | 20 (14) |
| Never smoked (%) | 77 | 81 | 81 |
| Mean exacerbations in previous year (± SD) | 2.2 (2.1) | 2.1 (2.2) | 2.1 (2.2) |
| High dose ICS use (%) | 50 | 52 | 89 |
| Pre-dose FEV1 (L) at baseline (± SD) | 1.84 (0.54) | 1.78 (0.60) | 1.58 (0.57) |
| Mean percent predicted FEV1 at baseline (%) (± SD) | 61 (11) | 58 (14) | 52 (15) |
| % Reversibility (± SD) | 27 (15) | 26 (22) | 19 (23) |
|
Atopic Medical History % Overall (AD %, NP %, AR %) |
73 (8, 11, 62) |
78 (10, 13, 69) |
72 (8, 21, 56) |
| Mean FeNO ppb (± SD) | 39 (35) | 35 (33) | 38 (31) |
| Mean total IgE IU/mL (± SD) | 435 (754) | 432 (747) | 431 (776) |
| Mean baseline blood Eosinophil count (± SD) cells/mcL | 350 (430) | 360 (370) | 350 (310) |
Exacerbations
AS Trials 1 and 2 evaluated the frequency of severe asthma exacerbations defined as deterioration of asthma requiring the use of systemic corticosteroids for at least 3 days or hospitalization or emergency room visit due to asthma that required systemic corticosteroids. In the primary analysis population (subjects with baseline blood eosinophil count of ≥300 cells/mcL in AS Trial 1 and the overall population in AS Trial 2), subjects receiving either Dupixent 200 mg or 300 mg Q2W had significant reductions in the rate of asthma exacerbations compared to placebo. In the overall population in AS Trial 2, the rate of severe exacerbations was 0.46 and 0.52 for Dupixent 200 mg Q2W and 300 mg Q2W, respectively, compared to matched placebo rates of 0.87 and 0.97. The rate ratio of severe exacerbations compared to placebo was 0.52 (95% CI: 0.41, 0.66) and 0.54 (95% CI: 0.43, 0.68) for Dupixent 200 mg Q2W and 300 mg Q2W, respectively. Results in subjects with baseline blood eosinophil counts ≥300 cells/mcL in AS Trials 1 and 2 are shown in Table 9.
Response rates by baseline blood eosinophils for AS Trial 2 are shown in Figure 3. Pre-specified subgroup analyses of AS Trials 1 and 2 demonstrated that there were greater reductions in severe exacerbations in subjects with higher baseline blood eosinophil levels. In AS Trial 2, reductions in exacerbations were significant in the subgroup of subjects with baseline blood eosinophils ≥150 cells/mcL. In subjects with baseline blood eosinophil count <150 cells/mcL, similar severe exacerbation rates were observed between Dupixent and placebo.
In AS Trial 2, the estimated rate ratio of exacerbations leading to hospitalizations and/or emergency room visits versus placebo was 0.53 (95% CI: 0.28, 1.03) and 0.74 (95% CI: 0.32, 1.70) with Dupixent 200 mg or 300 mg Q2W, respectively.
| Trial | Treatment |
Baseline Blood EOS ≥300 cells/mcL (primary analysis population, Trial 1) |
||
|---|---|---|---|---|
| N |
Rate (95% CI) |
Rate Ratio (95% CI) | ||
| AS Trial 1 |
Dupixent 200 mg Q2W |
65 |
0.30 (0.13, 0.68) |
0.29 (0.11, 0.76) |
|
Dupixent 300 mg Q2W |
64 |
0.20 (0.08, 0.52) |
0.19 (0.07, 0.56) |
|
| Placebo | 68 |
1.04 (0.57, 1.90) |
||
| AS Trial 2 |
Dupixent 200 mg Q2W |
264 |
0.37 (0.29, 0.48) |
0.34 (0.24, 0.48) |
| Placebo | 148 |
1.08 (0.85, 1.38) |
||
|
Dupixent 300 mg Q2W |
277 |
0.40 (0.32, 0.51) |
0.33 (0.23, 0.45) |
|
| Placebo | 142 |
1.24 (0.97, 1.57) |
||
Figure 3: Relative Risk in Annualized Event Rate of Severe Exacerbations across Baseline Blood Eosinophil Count (cells/mcL) in AS Trial 2
The time to first exacerbation was longer for the subjects receiving Dupixent compared to placebo in AS Trial 2 (Figure 4).
| *At the time of the database lock, not all patients had completed Week 52 |
| Figure 4: Kaplan Meier Incidence Curve for Time to First Severe Exacerbation in Subjects with Baseline Blood Eosinophils ≥300 cells/mcL (AS Trial 2)* |
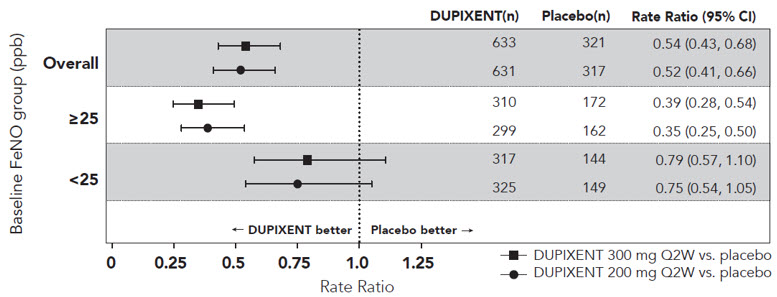
|
Lung Function
Significant increases in pre-bronchodilator FEV1 were observed at Week 12 for AS Trials 1 and 2 in the primary analysis populations (subjects with baseline blood eosinophil count of ≥300 cells/mcL in AS Trial 1 and the overall population in AS Trial 2). In the overall population in AS Trial 2, the FEV1 LS mean change from baseline was 0.32 L (21%) and 0.34 L (23%) for Dupixent 200 mg Q2W and 300 mg Q2W, respectively, compared to matched placebo means of 0.18 L (12%) and 0.21 L (14%). The mean treatment difference versus placebo was 0.14 L (95% CI: 0.08, 0.19) and 0.13 L (95% CI: 0.08, 0.18) for Dupixent 200 mg Q2W and 300 mg Q2W, respectively. Results in subjects with baseline blood eosinophil counts ≥300 cells/mcL in AS Trials 1 and 2 are shown in Table 10.
Improvements in FEV1 by baseline blood eosinophils for AS Trial 2 are shown in Figure 5. Subgroup analysis of AS Trials 1 and 2 demonstrated greater improvement in subjects with higher baseline blood eosinophils.
| Trial | Treatment |
Baseline Blood EOS ≥300 cells/mcL (primary analysis population, Trial 1) |
||
|---|---|---|---|---|
| N |
LS Mean Change from baseline L (%) |
LS Mean Difference vs. placebo (95% CI) |
||
| AS Trial 1 |
Dupixent 200 mg Q2W |
65 | 0.43 (25.9) |
0.26 (0.11, 0.40) |
|
Dupixent 300 mg Q2W |
64 | 0.39 (25.8) |
0.21 (0.06, 0.36) |
|
| Placebo | 68 | 0.18 (10.2) | ||
| AS Trial 2 |
Dupixent 200 mg Q2W |
264 | 0.43 (29.0) |
0.21 (0.13, 0.29) |
| Placebo | 148 | 0.21 (15.6) | ||
|
Dupixent 300 mg Q2W |
277 | 0.47 (32.5) |
0.24 (0.16, 0.32) |
|
| Placebo | 142 | 0.22 (14.4) | ||
Figure 5: LS Mean Difference in Change from Baseline vs Placebo to Week 12 in Pre-Bronchodilator FEV1 across Baseline Blood Eosinophil Counts (cells/mcL) in AS Trial 2
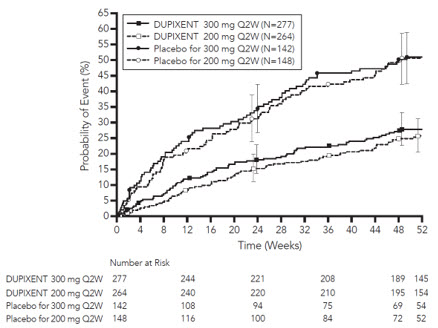
Mean changes in FEV1 over time in AS Trial 2 are shown in Figure 6.
Figure 6: Mean Change from Baseline in Pre-Bronchodilator FEV1 (L) Over Time in Subjects with Baseline Blood Eosinophils ≥300 cells/mcL (AS Trial 2)
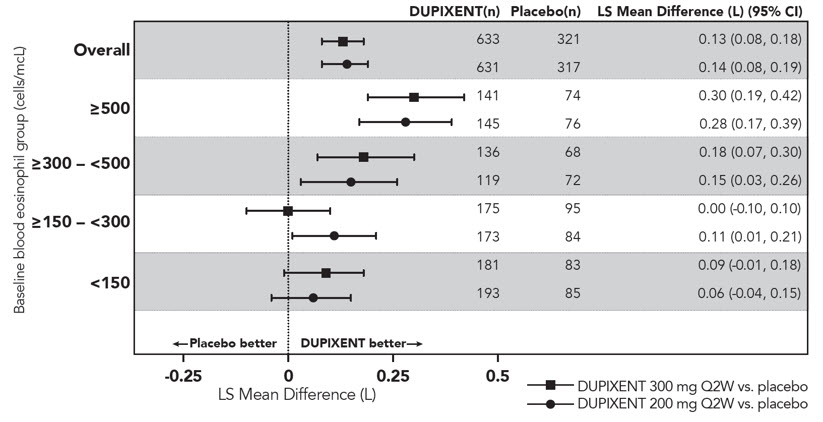
Additional Secondary Endpoints
ACQ-5 and AQLQ(S) were assessed in AS Trial 2 at 52 weeks. The responder rate was defined as an improvement in score of 0.5 or more (scale range 0-6 for ACQ-5 and 1-7 for AQLQ(S)).
The ACQ-5 responder rate for Dupixent 200 mg and 300 mg Q2W in the overall population was 69% vs 62% placebo (odds ratio 1.37; 95% CI: 1.01, 1.86) and 69% vs 63% placebo (odds ratio 1.28; 95% CI: 0.94, 1.73), respectively; and the AQLQ(S) responder rates were 62% vs 54% placebo (odds ratio 1.61; 95% CI: 1.17, 2.21) and 62% vs 57% placebo (odds ratio 1.33; 95% CI: 0.98, 1.81), respectively.The ACQ-5 responder rate for Dupixent 200 mg and 300 mg Q2W in subjects with baseline blood eosinophils ≥300 cells/mcL was 75% vs 67% placebo (odds ratio: 1.46; 95% CI: 0.90, 2.35) and 71% vs 64% placebo (odds ratio: 1.39; 95% CI: 0.88, 2.19), respectively; and the AQLQ(S) responder rates were 71% vs 55% placebo (odds ratio: 2.02; 95% CI: 1.24, 3.32) and 65% vs 55% placebo (odds ratio: 1.79; 95% CI: 1.13, 2.85), respectively.Oral Corticosteroid Reduction (AS Trial 3)
AS Trial 3 evaluated the effect of Dupixent on reducing the use of maintenance oral corticosteroids. The baseline mean oral corticosteroid dose was 12 mg in the placebo group and 11 mg in the group receiving Dupixent. The primary endpoint was the percent reduction from baseline of the final oral corticosteroid dose at Week 24 while maintaining asthma control.
Compared with placebo, subjects receiving Dupixent achieved greater reductions in daily maintenance oral corticosteroid dose, while maintaining asthma control. The mean percent reduction in daily OCS dose from baseline was 70% (median 100%) in subjects receiving Dupixent (95% CI: 60%, 80%) compared to 42% (median 50%) in subjects receiving placebo (95% CI: 33%, 51%). Reductions of 50% or higher in the OCS dose were observed in 82 (80%) subjects receiving Dupixent compared to 57 (53%) in those receiving placebo. The proportion of subjects with a mean final dose less than 5 mg at Weeks 24 was 72% for Dupixent and 37% for placebo (odds ratio 4.48 95% CI: 2.39, 8.39). A total of 54 (52%) subjects receiving Dupixent versus 31 (29%) subjects in the placebo group had a 100% reduction in their OCS dose.
In this 24-week trial, asthma exacerbations (defined as a temporary increase in oral corticosteroid dose for at least 3 days) were lower in subjects receiving Dupixent compared with those receiving placebo (annualized rate 0.65 and 1.60 for the Dupixent and placebo group, respectively; rate ratio 0.41 [95% CI 0.26, 0.63]) and improvement in pre-bronchodilator FEV1 from baseline to Week 24 was greater in subjects receiving Dupixent compared with those receiving placebo (LS mean difference for Dupixent versus placebo of 0.22 L [95% CI: 0.09 to 0.34 L]). Effects on lung function and on oral steroid and exacerbation reduction were similar irrespective of baseline blood eosinophil levels. The ACQ-5 and AQLQ(S) were also assessed in AS Trial 3 and showed improvements similar to those in AS Trial 2.
Chronic Rhinosinusitis with Nasal PolyposisThe chronic rhinosinusitis with nasal polyposis (CRSwNP) development program included two randomized, double-blind, parallel-group, multicenter, placebo-controlled studies (CSNP Trial 1 and CSNP Trial 2) in 724 subjects aged 18 years and older on background intranasal corticosteroids (INCS). These studies included subjects with CRSwNP despite prior sino-nasal surgery or treatment with, or who were ineligible to receive or were intolerant to, systemic corticosteroids in the past 2 years. Patients with chronic rhinosinusitis without nasal polyposis were not included in these trials. Rescue with systemic corticosteroids or surgery was allowed during the studies at the investigator's discretion. In CSNP Trial 1, a total of 276 subjects were randomized to receive either 300 mg Dupixent (N=143) or placebo (N=133) every other week for 24 weeks. In CSNP Trial 2, 448 subjects were randomized to receive either 300 mg Dupixent (N=150) every other week for 52 weeks, 300 mg Dupixent (N=145) every other week until week 24 followed by 300 mg Dupixent every 4 weeks until week 52, or placebo (N=153). All subjects had evidence of sinus opacification on the Lund Mackay (LMK) sinus CT scan and 73% to 90% of subjects had opacification of all sinuses. Subjects were stratified based on their histories of prior surgery and co-morbid asthma/nonsteroidal anti-inflammatory drug exacerbated respiratory disease (NSAID-ERD). A total of 63% of subjects reported previous sinus surgery, with a mean number of 2.0 prior surgeries, 74% used systemic corticosteroids in the previous 2 years with a mean number of 1.6 systemic corticosteroid courses in the previous 2 years, 59% had co-morbid asthma, and 28% had NSAID-ERD.
The co-primary efficacy endpoints were change from baseline to Week 24 in bilateral endoscopic nasal polyps score (NPS; 0-8 scale) as graded by central blinded readers, and change from baseline to Week 24 in nasal congestion/obstruction score averaged over 28 days (NC; 0-3 scale), as determined by subjects using a daily diary. For NPS, polyps on each side of the nose were graded on a categorical scale (0=no polyps; 1=small polyps in the middle meatus not reaching below the inferior border of the middle turbinate; 2=polyps reaching below the lower border of the middle turbinate; 3=large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle turbinate; 4=large polyps causing complete obstruction of the inferior nasal cavity). The total score was the sum of the right and left scores. Nasal congestion was rated daily by the subjects on a 0 to 3 categorical severity scale (0=no symptoms; 1=mild symptoms; 2=moderate symptoms; 3=severe symptoms).
In both studies, key secondary end-points at Week 24 included change from baseline in: LMK sinus CT scan score, daily loss of smell, and 22-item sino-nasal outcome test (SNOT-22). The LMK sinus CT scan score evaluated the opacification of each sinus using a 0 to 2 scale (0=normal; 1=partial opacification; 2=total opacification) deriving a maximum score of 12 per side and a total maximum score of 24 (higher scores indicate more opacification). Loss of smell was scored reflectively by the patient every morning on a 0-3 scale (0=no symptoms, 1=mild symptoms, 2=moderate symptoms, 3=severe symptoms). SNOT-22 includes 22 items assessing symptoms and symptom impact associated with CRSwNP with each item scored from 0 (no problem) to 5 (problem as bad as it can be) with a global score ranging from 0 to 110. SNOT-22 had a 2 week recall period. In the pooled efficacy results, the reduction in the proportion of subjects rescued with systemic corticosteroids and/or sino-nasal surgery (up to Week 52) were evaluated.
The demographics and baseline characteristics of these 2 trials are provided in Table 11 below.
| Parameter |
CSNP Trial 1 (N=276) |
CSNP Trial 2 (N=448) |
|---|---|---|
| SD = standard deviation; AM = morning; NPS = nasal polyps score; SNOT-22 = 22-item sino-nasal outcome test; NSAID-ERD = asthma/nonsteroidal anti-inflammatory drug exacerbated respiratory disease | ||
| *Higher scores indicate greater disease severity | ||
| Mean age (years) (SD) | 50 (13) | 52 (12) |
| % Male | 57 | 62 |
| Mean CRSwNP duration (years) (SD) | 11 (9) | 11 (10) |
| Patients with ≥ 1 prior surgery (%) | 72 | 58 |
| Patients with systemic corticosteroid use in the previous 2 years (%) | 65 | 80 |
| Mean Bilateral endoscopic NPS* (SD), range 0-8 | 5.8 (1.3) | 6.1 (1.2) |
| Mean Nasal congestion (NC) score* (SD), range 0-3 | 2.4 (0.6) | 2.4 (0.6) |
| Mean LMK sinus CT total score* (SD), range 0-24 | 19 (4.4) | 18 (3.8) |
| Mean loss of smell score* (AM), (SD) range 0-3 | 2.7 (0.5) | 2.8 (0.5) |
| Mean SNOT-22 total score* (SD), range 0-110 | 49.4 (20.2) | 51.9 (20.9) |
| Mean blood eosinophils (cells/mcL) (SD) | 440 (330) | 430 (350) |
| Mean total IgE IU/mL (SD) | 212 (276) | 240 (342) |
|
Atopic Medical History % Overall |
75% | 82% |
| Asthma (%) | 58 | 60 |
| NSAID-ERD (%) | 30 | 27 |
Clinical Response (CSNP Trial 1 and CSNP Trial 2)
The results for primary endpoints in CRSwNP studies are presented in Table 12.
| CSNP Trial 1 | CSNP Trial 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Placebo (n=133) |
Dupixent 300 mg Q2W (n=143) |
LS mean difference vs. Placebo (95% CI) |
Placebo (n=153) |
Dupixent 300 mg Q2W (n=295) |
LS mean difference vs. Placebo (95% CI) |
|||||
| Primary Endpoints at Week 24 | ||||||||||
| Scores | Baseline mean | LS mean change | Baseline mean | LS mean change | Baseline mean | LS mean change | Baseline mean | LS mean change | ||
|
A reduction in score indicates improvement. NPS = nasal polyps score; NC = nasal congestion/obstruction |
||||||||||
| NPS | 5.86 | 0.17 | 5.64 | -1.89 |
-2.06 (-2.43, -1.69) |
5.96 | 0.10 | 6.18 | -1.71 |
-1.80 (-2.10, -1.51) |
| NC | 2.45 | -0.45 | 2.26 | -1.34 |
-0.89 (-1.07, -0.71) |
2.38 | -0.38 | 2.46 | -1.25 |
-0.87 (-1.03, -0.71) |
Statistically significant efficacy was observed in CSNP Trial 2 with regard to improvement in bilateral endoscopic NPS score at week 24 and week 52 (see Figure 7).
Figure 7: LS Mean Change from Baseline in Bilateral Nasal Polyps Score (NPS) up to Week 52 in CSNP Trial 2 - ITT Population
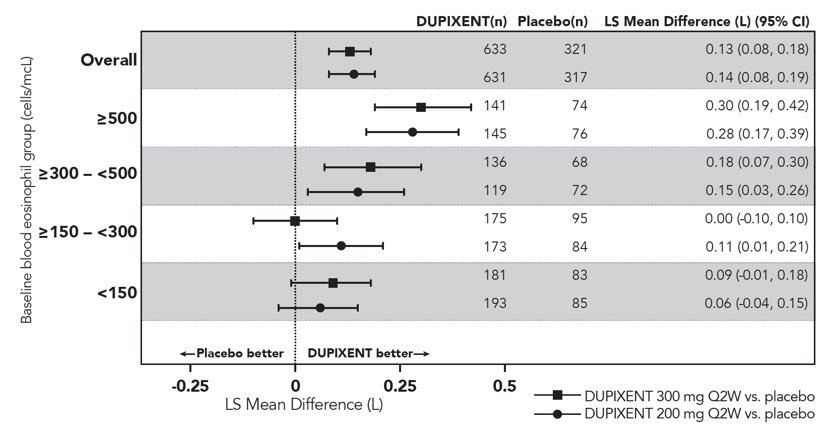
Similar results were seen in CSNP Trial 1 at Week 24. In the post-treatment period when subjects were off Dupixent, the treatment effect diminished over time (see Figure 8).
Figure 8: LS Mean Change from Baseline in Bilateral Nasal Polyps Score (NPS) up to Week 48 in CSNP Trial 1 - ITT Population
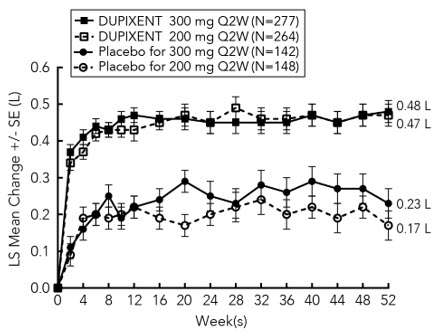
At Week 52, the LS mean difference for nasal congestion in the Dupixent group versus placebo was -0.98 (95% CI -1.17, -0.79). In both studies, significant improvements in nasal congestion were observed as early as the first assessment at Week 4. The LS mean difference for nasal congestion at Week 4 in the Dupixent group versus placebo was -0.41 (95% CI: -0.52, -0.30) in CSNP Trial 1 and -0.37 (95% CI: -0.46, -0.27) in CSNP Trial 2.
A significant decrease in the LMK sinus CT scan score was observed. The LS mean difference for LMK sinus CT scan score at Week 24 in the Dupixent group versus placebo was -7.44 (95% CI: -8.35, -6.53) in CSNP Trial 1 and -5.13 (95% CI: -5.80, -4.46) in CSNP Trial 2. At Week 52, in CSNP Trial 2 the LS mean difference for LMK sinus CT scan score in the Dupixent group versus placebo was -6.94 (95% CI: -7.87, -6.01).
Dupilumab significantly improved the loss of smell compared to placebo. The LS mean difference for loss of smell at Week 24 in the Dupixent group versus placebo was -1.12 (95% CI: -1.31, -0.93) in CSNP Trial 1 and -0.98 (95% CI: -1.15, -0.81) in CSNP Trial 2. At Week 52, the LS mean difference for loss of smell in the Dupixent group versus placebo was -1.10 (95% CI -1.31, -0.89). In both studies, significant improvements in daily loss of smell severity were observed as early as the first assessment at Week 4.
Dupilumab significantly decreased sino-nasal symptoms as measured by SNOT-22 compared to placebo. The LS mean difference for SNOT-22 at Week 24 in the Dupixent group versus placebo was -21.12 (95% CI: -25.17, -17.06) in CSNP Trial 1 and -17.36 (95% CI: -20.87, -13.85) in CSNP Trial 2. At Week 52, the LS mean difference in the Dupixent group versus placebo was -20.96 (95% CI -25.03, -16.89).
In the pre-specified multiplicity-adjusted pooled analysis of two studies, treatment with Dupixent resulted in significant reduction of systemic corticosteroid use and need for sino-nasal surgery versus placebo (HR of 0.24; 95% CI: 0.17, 0.35) (see Figure 9). The proportion of subjects who required systemic corticosteroids was reduced by 74% (HR of 0.26; 95% CI: 0.18, 0.38). The total number of systemic corticosteroid courses per year was reduced by 75% (RR of 0.25; 95% CI: 0.17, 0.37). The proportion of subjects who required surgery was reduced by 83% (HR of 0.17; 95% CI: 0.07, 0.46).
Figure 9: Kaplan Meier Curve for Time to First Systemic Corticosteroid Use and/or Sino-Nasal Surgery During Treatment Period - ITT population CSNP Trial 1 and CSNP Trial 2 Pooled
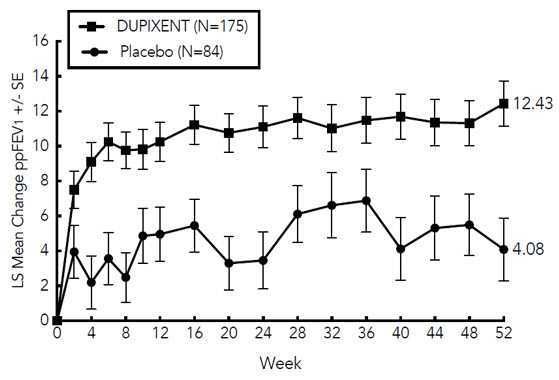
The effects of Dupixent on the primary endpoints of NPS and nasal congestion and the key secondary endpoint of LMK sinus CT scan score were consistent in patients with prior surgery and without prior surgery.
In subjects with co-morbid asthma, improvements in pre-bronchodilator FEV1 were similar to patients in the asthma program.
How Supplied/Storage and HandlingHow SuppliedDupixent (dupilumab) Injection is a clear to slightly opalescent, colorless to pale yellow solution, supplied in single-dose pre-filled syringes with needle shield. Each pre-filled syringe with needle shield is designed to deliver either 300 mg of Dupixent in 2 mL (NDC 0024-5914-00) or 200 mg of Dupixent in 1.14 mL solution (NDC 0024-5918-00).
Dupixent is available in cartons containing 2 pre-filled syringes with needle shield.
| Pack Size | 300 mg/2 mL Pre-filled Syringe with Needle Shield | 200 mg/1.14 mL Pre-filled Syringe with Needle Shield |
|---|---|---|
| Pack of 2 syringes | NDC 0024-5914-01 | NDC 0024-5918-01 |
Dupixent is sterile and preservative-free. Discard any unused portion.
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
If necessary, pre-filled syringes may be kept at room temperature up to 77°F (25°C) for a maximum of 14 days. Do not store above 77°F (25°C). After removal from the refrigerator, Dupixent must be used within 14 days or discarded.
Do not expose the pre-filled syringe to heat or direct sunlight.
Do NOT freeze. Do NOT shake.
Patient Counseling InformationAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Pregnancy Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Dupixent during pregnancy. Encourage participation in the registry. [see Use in Specific Populations (8.1)].
Administration Instructions
Provide proper training to patients and/or caregivers on proper subcutaneous injection technique, including aseptic technique, and the preparation and administration of Dupixent prior to use. Advise patients to follow sharps disposal recommendations [see Instructions for Use].
Hypersensitivity
Advise patients to discontinue Dupixent and to seek immediate medical attention if they experience any symptoms of systemic hypersensitivity reactions [see Warnings and Precautions (5.1)].
Conjunctivitis and Keratitis
Advise patients to consult their healthcare provider if new onset or worsening eye symptoms develop [see Warnings and Precautions (5.2)].
Eosinophilic Conditions
Advise patients to notify their healthcare provider if they present with clinical features of eosinophilic pneumonia or vasculitis consistent with eosinophilic granulomatosis with polyangiitis [see Warnings and Precautions (5.3)].
Not for Acute Asthma Symptoms or Deteriorating Disease
Inform patients that Dupixent does not treat acute asthma symptoms or acute exacerbations. Inform patients to seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with Dupixent [see Warnings and Precautions (5.4)].
Reduction in Corticosteroid Dosage
Inform patients to not discontinue systemic or inhaled corticosteroids except under the direct supervision of a physician. Inform patients that reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy [see Warnings and Precautions (5.5)].
Patients with Co-morbid Asthma
Advise patients with atopic dermatitis or CRSwNP who have co-morbid asthma not to adjust or stop their asthma treatment without talking to their physicians [see Warnings and Precautions (5.6)].
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License No. 1760
Marketed by:
sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and
Regeneron Pharmaceuticals, Inc. (Tarrytown, NY 10591)
Dupixent® is a registered trademark of Sanofi Biotechnology
© 2019 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
Revised: June 2019
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: June 2019 | ||
|
Patient Information |
|||
|
What is Dupixent? Dupixent is a prescription medicine used: to treat people aged 12 years and older with moderate-to-severe atopic dermatitis (eczema) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. Dupixent can be used with or without topical corticosteroids.with other asthma medicines for the maintenance treatment of moderate-to-severe asthma in people aged 12 years and older whose asthma is not controlled with their current asthma medicines. Dupixent helps prevent severe asthma attacks (exacerbations) and can improve your breathing. Dupixent may also help reduce the amount of oral corticosteroids you need while preventing severe asthma attacks and improving your breathing.with other medicines to treat chronic rhinosinusitis with nasal polyposis in adults whose disease is not controlled.Dupixent works by blocking two proteins that contribute to a type of inflammation that plays a major role in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis.Dupixent is not used to treat sudden breathing problems.It is not known if Dupixent is safe and effective in children with atopic dermatitis or asthma under 12 years of age.It is not known if Dupixent is safe and effective in children with chronic rhinosinusitis with nasal polyposis under 18 years of age. |
|||
|
Do not use Dupixent if you are allergic to dupilumab or to any of the ingredients in Dupixent. See the end of this leaflet for a complete list of ingredients in Dupixent. |
|||
|
Before using Dupixent, tell your healthcare provider about all your medical conditions, including if you: have eye problems.have a parasitic (helminth) infection.are taking oral, topical, or inhaled corticosteroid medicines. Do not stop taking your corticosteroid medicines unless instructed by your healthcare provider. This may cause other symptoms that were controlled by the corticosteroid medicine to come back.are scheduled to receive any vaccinations. You should not receive a "live vaccine" if you are treated with Dupixent.are pregnant or plan to become pregnant. It is not known whether Dupixent will harm your unborn baby.Pregnancy Registry. There is a pregnancy registry for women who take Dupixent during pregnancy. The purpose of this registry is to collect information about your health and your baby's health. You can talk to your healthcare provider or contact 1-877-311-8972 or go to https://mothertobaby.org/ongoing-study/Dupixent/ to enroll in this registry or get more information.are breastfeeding or plan to breastfeed. It is not known whether Dupixent passes into your breast milk. Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. If you have asthma and are taking asthma medicines, do not change or stop your asthma medicine without talking to your healthcare provider. |
|||
|
How should I use Dupixent? See the detailed "Instructions for Use" that comes with Dupixent for information on how to prepare and inject Dupixent and how to properly store and throw away (dispose of) used Dupixent pre-filled syringes.Use Dupixent exactly as prescribed by your healthcare provider.Dupixent comes as a single-dose pre-filled syringe with needle shield.Dupixent is given as an injection under the skin (subcutaneous injection).If your healthcare provider decides that you or a caregiver can give the injections of Dupixent, you or your caregiver should receive training on the right way to prepare and inject Dupixent. Do not try to inject Dupixent until you have been shown the right way by your healthcare provider. In children 12 years of age and older, it is recommended that Dupixent be given by or under the supervision of an adult.If you miss a dose of Dupixent, give the injection within 7 days from the missed dose, then continue with the original schedule. If the missed dose is not given within 7 days, wait until the next scheduled dose to give your Dupixent injection.If you inject more Dupixent than prescribed, call your healthcare provider right away.Your healthcare provider may prescribe other medicines to use with Dupixent. Use the other prescribed medicines exactly as your healthcare provider tells you to. |
|||
|
What are the possible side effects of Dupixent? Dupixent can cause serious side effects, including: Allergic reactions (hypersensitivity), including a severe reaction known as anaphylaxis. Stop using Dupixent and tell your healthcare provider or get emergency help right away if you get any of the following symptoms: |
|||
| breathing problemsfevergeneral ill feelingswollen lymph nodes | swelling of the face, mouth, and tonguehivesitching | fainting, dizziness, feeling lightheaded (low blood pressure)joint painskin rash | |
| Eye problems. Tell your healthcare provider if you have any new or worsening eye problems, including eye pain or changes in vision.Inflammation of your blood vessels. Rarely, this can happen in people with asthma who receive Dupixent. This may happen in people who also take a steroid medicine by mouth that is being stopped or the dose is being lowered. It is not known whether this is caused by Dupixent. Tell your healthcare provider right away if you have: | |||
| rashshortness of breathpersistent fever | chest paina feeling of pins and needles or numbness of your arms or legs | ||
|
The most common side effects of Dupixent include: injection site reactionseye and eyelid inflammation, including redness, swelling, and itchingpain in the throat (oropharyngeal pain)cold sores in your mouth or on your lipshigh count of a certain white blood cell (eosinophilia)trouble sleeping (insomnia)toothachegastritisjoint pain (arthralgia)Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Dupixent. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
General information about the safe and effective use of Dupixent. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Dupixent for a condition for which it was not prescribed. Do not give Dupixent to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Dupixent that is written for health professionals. |
|||
|
What are the ingredients in Dupixent? Active ingredient: dupilumab Inactive ingredients: L-arginine hydrochloride, L-histidine, polysorbate 80, sodium acetate, sucrose, and water for injection. |
|||
|
REGENERON SANOFI GENZYME Manufactured by: Regeneron Pharmaceuticals, Inc., Tarrytown, NY 10591 U.S. License No. 1760 Marketed by: sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY 10591) Dupixent® is a registered trademark of Sanofi Biotechnology / © 2019 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved. For more information about Dupixent, go to www.Dupixent.com or call 1-844-Dupixent (1-844-387-4936). |
|||
Read this Instructions for Use before using the Dupixent Pre-filled Syringe. Do not inject yourself or someone else until you have been shown how to inject Dupixent. In adolescents 12 years of age and older, it is recommended that Dupixent be administered by or under supervision of an adult. Your healthcare provider can show you or your caregiver how to prepare and inject a dose of Dupixent before you try to do it yourself the first time. Keep these instructions for future use. Call your healthcare provider if you have any questions.
This device is a Single-Dose Pre-filled Syringe (called "Dupixent Syringe" in these instructions). It contains 300 mg of Dupixent for injection under the skin (subcutaneous injection).
The parts of the Dupixent Syringe are shown below:
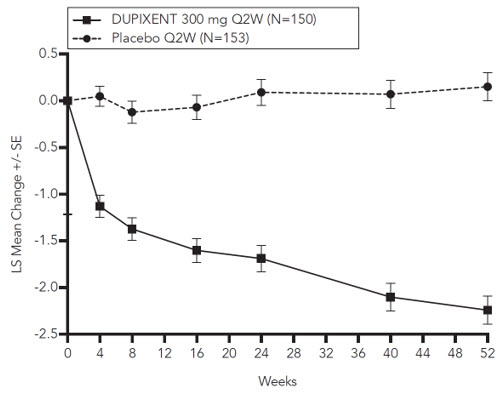
| Important Information | |
| Read all of the instructions carefully before using the Dupixent Syringe.Ask your healthcare provider how often you will need to inject the medicine.Rotate the injection site each time you inject.Do not use the Dupixent Syringe if it has been dropped on a hard surface or damaged.Do not use the Dupixent Syringe if the Needle Cap is missing or not securely attached.Do not touch the Plunger Rod until you are ready to inject.Do not inject through clothes.Do not get rid of any air bubble in the Dupixent Syringe. | To reduce the risk of accidental needle sticks, each pre-filled syringe has a Needle Shield that is automatically activated to cover the needle after you have given your injection.Do not pull back on the Plunger Rod at any time.Do not remove the Needle Cap until just before you give the injection.Throw away (dispose of) the used Dupixent Single-Dose Pre-filled Syringe right away after use. See "Step 13: Dispose" below.Do not re-use a Dupixent Single-Dose Pre-filled Syringe. |
| How should I store Dupixent?Keep Dupixent Syringes and all medicines out of the reach of children.Store Dupixent Syringes in the refrigerator between 36°F and 46°F (2°C and 8°C).Store Dupixent Syringes in the original carton to protect them from light.Dupixent Syringes can be stored at room temperature up to 77°F (25°C) up to 14 days. Throw away (dispose of) any Dupixent Syringes that have been left at room temperature for longer than 14 days.Do not shake the Dupixent Syringe.Do not heat the Dupixent Syringe.Do not freeze the Dupixent Syringe.Do not put the Dupixent Syringe into direct sunlight. | |
Step 1: Remove
Remove the Dupixent Syringe from the carton by holding the middle of the Syringe Body.
 Do not pull off the Needle Cap until you are ready to inject.
Do not pull off the Needle Cap until you are ready to inject.
 Do not use the Dupixent Syringe if it has been dropped on a hard surface or damaged.
Do not use the Dupixent Syringe if it has been dropped on a hard surface or damaged.

Step 2: Prepare
Ensure you have the following:
the Dupixent Pre-filled Syringe1 alcohol wipe*1 cotton ball or gauze*a sharps disposal container* (See Step 13)*Items not included in the carton
Step 3: Check
When you receive your Dupixent Syringes, always check to see that:
you have the correct medicine and dose.the expiration date on the Single-Dose Pre-filled Syringe has not passed.
 Do not use the Dupixent Syringe if the expiration date has passed.
Do not use the Dupixent Syringe if the expiration date has passed.
Step 4: Inspect
Look at the medicine through the Viewing Window on the Dupixent Syringe:
Check to see if the liquid is clear and colorless to pale yellow.
Note: You may see an air bubble, this is normal.
 Do not use the Dupixent Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
Do not use the Dupixent Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
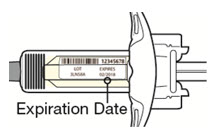
Step 5: Wait 45 minutes
Lay the Dupixent Syringe on a flat surface and let it naturally warm to room temperature for at least 45 minutes.
 Do not heat the Dupixent Syringe.
Do not heat the Dupixent Syringe.
 Do not put the Dupixent Syringe into direct sunlight.
Do not put the Dupixent Syringe into direct sunlight.
 Do not keep Dupixent Syringes at room temperature for more than 14 days. Throw away (dispose of) any Dupixent Syringes that have been left at room temperature for longer than 14 days.
Do not keep Dupixent Syringes at room temperature for more than 14 days. Throw away (dispose of) any Dupixent Syringes that have been left at room temperature for longer than 14 days.
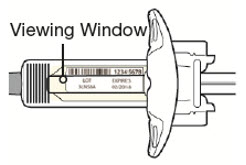
Step 6: Choose your injection site
You can inject into your thigh or stomach, except for the 2 inches (5 cm) around your belly button (navel).If a caregiver injects your dose, they can also use the outer area of the upper arm.Choose a different site each time you inject Dupixent.
 Do not inject into skin that is tender, damaged, bruised or scarred.
Do not inject into skin that is tender, damaged, bruised or scarred.
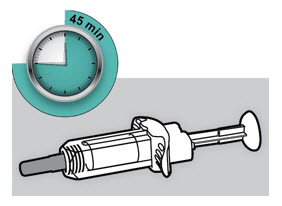
Step 7: Clean
Wash your hands.
Clean the injection site with an alcohol wipe.
Let your skin dry before injecting.
 Do not touch the injection site again or blow on it before the injection.
Do not touch the injection site again or blow on it before the injection.
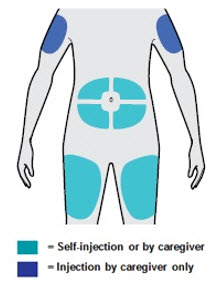
Step 8: Remove Needle Cap
Hold the Dupixent Syringe in the middle of the Syringe Body with the Needle pointing away from you and pull off the Needle Cap.
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.
 Do not touch the Needle.
Do not touch the Needle.
Inject your medicine right away after removing the Needle Cap.
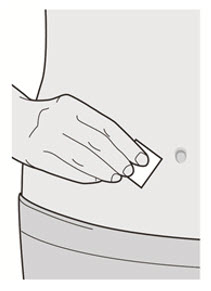
Step 9: Pinch
Pinch a fold of skin at the injection site (thigh or stomach, except 2 inches around your belly button, or outer area of the upper arm if injected by your caregiver). The figure below shows an example of pinching a fold of skin on your stomach.
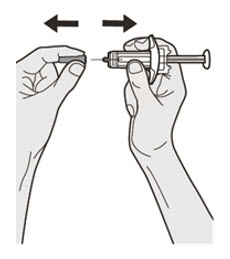
Step 10: Insert
Insert the Needle completely into the fold of the skin at about a 45° angle.
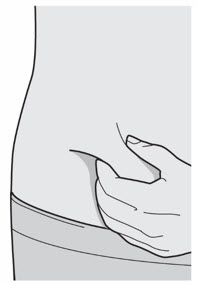
Step 11: Push
Relax the pinch.
Push the Plunger Rod down slowly and steadily as far as it will go until the Dupixent Syringe is empty.
Note: You will feel some resistance. This is normal.
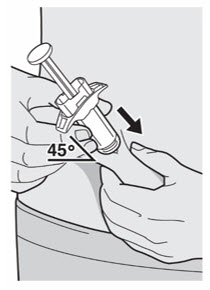
Step 12: Release and Remove
Lift your thumb to release the Plunger Rod until the Needle is covered by the Needle Shield and then remove the Syringe from the injection site.
Lightly press a cotton ball or gauze on the injection site if you see any blood.
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.
 Do not rub your skin after the injection.
Do not rub your skin after the injection.
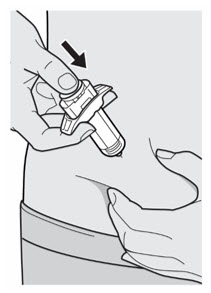
Step 13: Dispose
Put your used Needles, Dupixent Syringes, and Needle Caps in a FDA-cleared sharps disposal container right away after use.
 Do not dispose of (throw away) Needles, Dupixent Syringes, and Needle Caps in your household trash.
Do not dispose of (throw away) Needles, Dupixent Syringes, and Needle Caps in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
made of a heavy-duty plastic,can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,upright and stable during use,leak-resistant, andproperly labeled to warn of hazardous waste inside the containerWhen your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Needles and Syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.
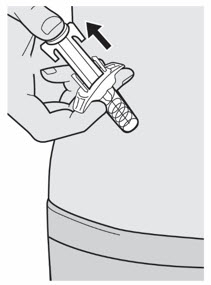
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
REGENERON SANOFI GENZYME
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License No. 1760
Marketed by:
sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and
Regeneron Pharmaceuticals, Inc. (Tarrytown, NY 10591)
Dupixent® is a registered trademark of Sanofi Biotechnology
© 2019 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
Issue Date: March 2019
Instructions for UseRead this Instructions for Use before using the Dupixent Pre-filled Syringe. Do not inject yourself or someone else until you have been shown how to inject Dupixent. In adolescents 12 years of age and older, it is recommended that Dupixent be administered by or under supervision of an adult. Your healthcare provider can show you or your caregiver how to prepare and inject a dose of Dupixent before you try to do it yourself the first time. Keep these instructions for future use. Call your healthcare provider if you have any questions.
This device is a Single-Dose Pre-filled Syringe (called "Dupixent Syringe" in these instructions). It contains 200 mg of Dupixent for injection under the skin (subcutaneous injection).
The parts of the Dupixent Syringe are shown below:
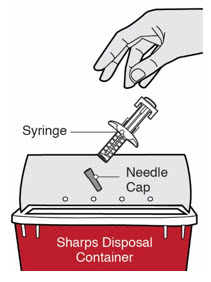
| Important Information | |
| Read all of the instructions carefully before using the Dupixent Syringe.Ask your healthcare provider how often you will need to inject the medicine.Rotate the injection site each time you inject.Do not use the Dupixent Syringe if it has been dropped on a hard surface or damaged.Do not use the Dupixent Syringe if the Needle Cap is missing or not securely attached.Do not touch the Plunger Rod until you are ready to inject.Do not inject through clothes.Do not get rid of any air bubble in the Dupixent Syringe. | To reduce the risk of accidental needle sticks, each pre-filled syringe has a Needle Shield that is automatically activated to cover the needle after you have given your injection.Do not pull back on the Plunger Rod at any time.Do not remove the Needle Cap until just before you give the injection.Throw away (dispose of) the used Dupixent Single-Dose Pre-filled Syringe right away after use. See "Step 13: Dispose" below.Do not re-use a Dupixent Single-Dose Pre-filled Syringe. |
| How should I store Dupixent?Keep Dupixent Syringes and all medicines out of the reach of children.Store Dupixent Syringes in the refrigerator between 36°F and 46°F (2°C and 8°C).Store Dupixent Syringes in the original carton to protect them from light.Dupixent Syringes can be stored at room temperature up to 77°F (25°C) up to 14 days. Throw away (dispose of) any Dupixent Syringes that have been left at room temperature for longer than 14 days.Do not shake the Dupixent Syringe.Do not heat the Dupixent Syringe.Do not freeze the Dupixent Syringe.Do not put the Dupixent Syringe into direct sunlight. | |
Step 1: Remove
Remove the Dupixent Syringe from the carton by holding the middle of the Syringe Body.
 Do not pull off the Needle Cap until you are ready to inject.
Do not pull off the Needle Cap until you are ready to inject.
 Do not use the Dupixent Syringe if it has been dropped on a hard surface or damaged.
Do not use the Dupixent Syringe if it has been dropped on a hard surface or damaged.
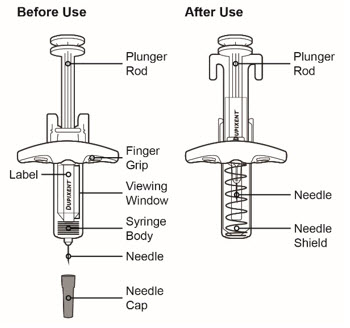
Step 2: Prepare
Ensure you have the following:
the Dupixent Pre-filled Syringe1 alcohol wipe*1 cotton ball or gauze*a sharps disposal container* (See Step 13)*Items not included in the carton

Step 3: Check
When you receive your Dupixent Syringes, always check to see that:
you have the correct medicine and dose.the expiration date on the Single-Dose Pre-filled Syringe has not passed.
 Do not use the Dupixent Syringe if the expiration date has passed.
Do not use the Dupixent Syringe if the expiration date has passed.
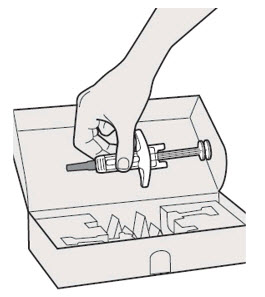
Step 4: Inspect
Look at the medicine through the Viewing Window on the Dupixent Syringe:
Check to see if the liquid is clear and colorless to pale yellow.
Note: You may see an air bubble, this is normal.
 Do not use the Dupixent Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
Do not use the Dupixent Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.

Step 5: Wait 30 minutes
Lay the Dupixent Syringe on a flat surface and let it naturally warm to room temperature for at least 30 minutes.
 Do not heat the Dupixent Syringe.
Do not heat the Dupixent Syringe.
 Do not put the Dupixent Syringe into direct sunlight.
Do not put the Dupixent Syringe into direct sunlight.
 Do not keep Dupixent Syringes at room temperature for more than 14 days. Throw away (dispose of) any Dupixent Syringes that have been left at room temperature for longer than 14 days.
Do not keep Dupixent Syringes at room temperature for more than 14 days. Throw away (dispose of) any Dupixent Syringes that have been left at room temperature for longer than 14 days.
Step 6: Choose your injection site
You can inject into your thigh or stomach, except for the 2 inches (5 cm) around your belly button (navel).If a caregiver injects your dose, they can also use the outer area of the upper arm.Choose a different site each time you inject Dupixent.
 Do not inject into skin that is tender, damaged, bruised or scarred.
Do not inject into skin that is tender, damaged, bruised or scarred.
Step 7: Clean
Wash your hands.
Clean the injection site with an alcohol wipe.
Let your skin dry before injecting.
 Do not touch the injection site again or blow on it before the injection.
Do not touch the injection site again or blow on it before the injection.
Step 8: Remove Needle Cap
Hold the Dupixent Syringe in the middle of the Syringe Body with the Needle pointing away from you and pull off the Needle Cap.
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.
 Do not touch the Needle.
Do not touch the Needle.
Inject your medicine right away after removing the Needle Cap.
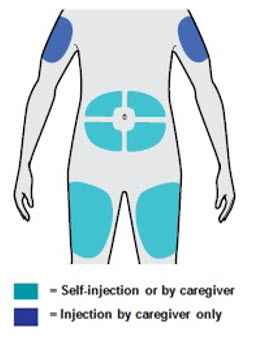
Step 9: Pinch
Pinch a fold of skin at the injection site (thigh or stomach, except 2 inches around your belly button, or outer area of the upper arm if injected by your caregiver). The figure below shows an example of pinching a fold of skin on your stomach.
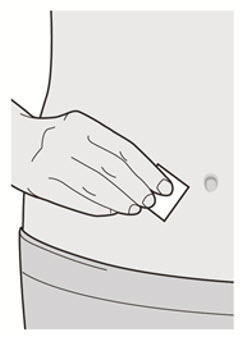
Step 10: Insert
Insert the Needle completely into the fold of the skin at about a 45° angle.
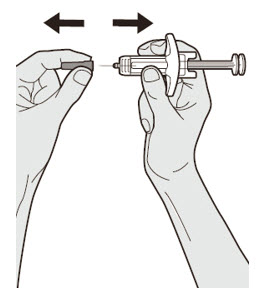
Step 11: Push
Relax the pinch.
Push the Plunger Rod down slowly and steadily as far as it will go until the Dupixent Syringe is empty.
Note: You will feel some resistance. This is normal.
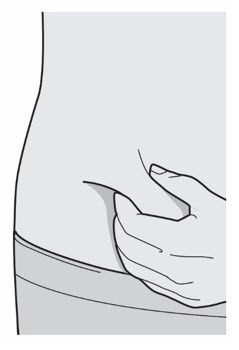
Step 12: Release and Remove
Lift your thumb to release the Plunger Rod until the Needle is covered by the Needle Shield and then remove the Syringe from the injection site.
Lightly press a cotton ball or gauze on the injection site if you see any blood.
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.
 Do not rub your skin after the injection.
Do not rub your skin after the injection.
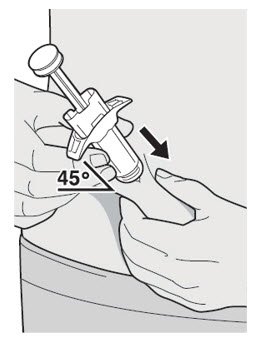
Step 13: Dispose
Put your used Needles, Dupixent Syringes, and Needle Caps in a FDA-cleared sharps disposal container right away after use.
 Do not dispose of (throw away) Needles, Dupixent Syringes, and Needle Caps in your household trash.
Do not dispose of (throw away) Needles, Dupixent Syringes, and Needle Caps in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
made of a heavy-duty plastic,can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,upright and stable during use,leak-resistant, andproperly labeled to warn of hazardous waste inside the containerWhen your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Needles and Syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.
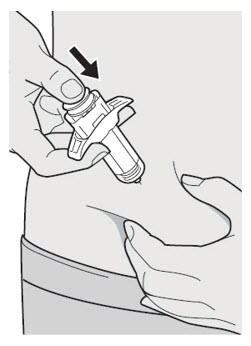
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
REGENERON SANOFI GENZYME
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License No. 1760
Marketed by:
sanofi-aventis U.S. LLC (Bridgewater, NJ 08807) and
Regeneron Pharmaceuticals, Inc. (Tarrytown, NY 10591)
Dupixent® is a registered trademark of Sanofi Biotechnology
© 2019 Regeneron Pharmaceuticals, Inc. / sanofi-aventis U.S. LLC. All rights reserved.
Issue Date: March 2019
PRINCIPAL DISPLAY PANEL - 300 mg/2 mL Syringe Carton - 5914NDC 0024-5914-01
Rx Only
Dupixent®
(dupilumab)
Injection
300 mg/2 mL (150 mg/mL)
For Subcutaneous Use Only.
300 mg/2 mL
2
Single-dose
Pre-filled Syringes
with Needle Shield
Keep out of reach of children. Do not use after
expiration. Do not use if seal is broken or damaged.
Store refrigerated at 36° to 46°F (2° to 8°C) in the original carton.
Dosage and Administration: See package insert for dosage information and directions for use.
SANOFI GENZYME
REGENERON
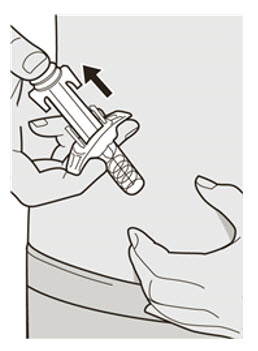
NDC 0024-5916-01
Rx Only
Dupixent®
(dupilumab)
Injection
300 mg/2 mL (150 mg/mL)
For Subcutaneous Injection Only.
300 mg/2 mL
2
Single-dose
Pre-filled Syringes
Keep out of reach of children. Do not use after
expiration. Do not use if seal is broken or damaged.
Store refrigerated at 36° to 46°F (2° to 8°C) in the original carton.
Dosage and Administration: See package insert for dosage information and directions for use.
SANOFI GENZYME
REGENERON
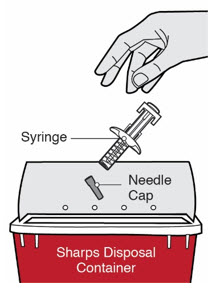
NDC 0024-5918-01
Rx Only
Dupixent®
(dupilumab)
Injection
200 mg/1.14 mL (175 mg/mL)
For Subcutaneous Use Only.
200 mg/1.14 mL
2
Single-dose
Pre-filled Syringes
with Needle Shield
Keep out of reach of children. Do not use after
expiration. Do not use if seal is broken or damaged.
Store refrigerated at 36° to 46°F (2° to 8°C) in the original carton.
Dosage and Administration: See package insert for dosage information and directions for use.
SANOFI GENZYME
REGENERON
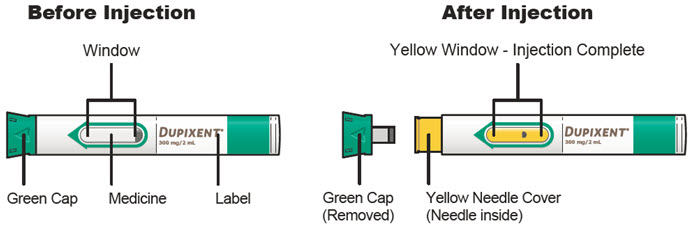
|
Dupixent dupilumab injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
Dupixent dupilumab injection, solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
Dupixent dupilumab injection, solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Regeneron Pharmaceuticals, Inc. | 945589711 | API MANUFACTURE(0024-5914, 0024-5916, 0024-5918), ANALYSIS(0024-5914, 0024-5916, 0024-5918) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi-Winthrop Industrie | 297730488 | ANALYSIS(0024-5914, 0024-5916, 0024-5918), MANUFACTURE(0024-5914, 0024-5916, 0024-5918), LABEL(0024-5914, 0024-5916, 0024-5918), PACK(0024-5914, 0024-5916, 0024-5918) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi-Aventis Deutschland GmbH | 313218430 | ANALYSIS(0024-5914, 0024-5916, 0024-5918) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Catalent Indiana, LLC | 172209277 | ANALYSIS(0024-5914, 0024-5916, 0024-5918), LABEL(0024-5914, 0024-5916, 0024-5918), PACK(0024-5914, 0024-5916, 0024-5918), MANUFACTURE(0024-5914, 0024-5916, 0024-5918) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Regeneron Ireland Unlimited Company | 985528196 | API MANUFACTURE(0024-5914, 0024-5916, 0024-5918), ANALYSIS(0024-5914, 0024-5916, 0024-5918) | |