

SIMPONI 戈利木单抗预装注射剂




| 通用中文 | 戈利木单抗预装注射剂 | 通用外文 | Golimumab Prefilled Syringe |
| 品牌中文 | 欣普尼 | 品牌外文 | SIMPONI |
| 其他名称 | 靶点TNF-α | ||
| 公司 | 杨森(Janssen-Cilag) | 产地 | 荷兰(Netherlands) |
| 含量 | 50mg /0.5ml | 包装 | 1支/盒 |
| 剂型给药 | 针剂 注射 | 储存 | 2度-8度(冰箱冷藏,禁止冷冻) |
| 适用范围 | 免疫性疾病 关节炎 风湿病 类风湿关节炎(RA) 银屑病关节炎(PSA),强直性脊柱炎(AS) | ||
| 通用中文 | 戈利木单抗预装注射剂 |
| 通用外文 | Golimumab Prefilled Syringe |
| 品牌中文 | 欣普尼 |
| 品牌外文 | SIMPONI |
| 其他名称 | 靶点TNF-α |
| 公司 | 杨森(Janssen-Cilag) |
| 产地 | 荷兰(Netherlands) |
| 含量 | 50mg /0.5ml |
| 包装 | 1支/盒 |
| 剂型给药 | 针剂 注射 |
| 储存 | 2度-8度(冰箱冷藏,禁止冷冻) |
| 适用范围 | 免疫性疾病 关节炎 风湿病 类风湿关节炎(RA) 银屑病关节炎(PSA),强直性脊柱炎(AS) |
戈利木单抗注射液说明书
请仔细阅读说明书并在医师指导下使用。
警告:严重感染和恶性肿瘤
严重感染
使用本品进行治疗的患者发生严重感染的风险增高,可导致住院或死亡。发生这类
感染的多数患者合并使用免疫抑制剂,例如甲氨蝶呤或糖皮质激素。
如果患者发生严重感染,应停用本品。
使用肿瘤坏死因子(TNF)阻滞剂(包括本品)报告的感染包括:
•
•
活动性结核病,包括潜伏性结核的再激活。结核病患者常出现播散性或肺外疾病。
需在本品治疗前及治疗期间进行潜伏性结核检测。在本品用药前对潜伏性结核进行
治疗。
侵袭性真菌感染,包括:组织胞浆菌病、球孢子菌病、念珠菌病、曲霉病、芽生菌
病和肺孢子菌病。患组织胞浆菌病或其他侵袭性真菌感染的患者可出现播散性而非
局灶性的感染。在某些活动性感染的患者中,组织胞浆菌病的抗原和抗体检测结果
可为阴性。对于存在侵袭性真菌感染风险、发生重度系统性疾病的患者,应考虑进
行经验性抗真菌治疗。
•
机会性感染(包括军团菌属、李斯特菌属等细菌感染、病毒感染或其他机会性感染)。
慢性或复发性感染患者治疗前,应慎重考虑本品治疗的风险和获益。
在本品治疗期间及治疗后,应密切监测患者是否出现感染的症状和体征,包括开始
治疗前潜伏性结核感染检测结果阴性的患者发生结核病的可能。
恶性肿瘤
本品在中国尚未批准用于儿童和青少年患者,但国外相关研究数据和上市后资料中
提示儿童和青少年患者使用包括本品在内的 TNF阻滞剂治疗时,已报告有淋巴肿瘤和其
他恶性肿瘤发生(部分为致死性)。
【药品名称】
通用名称:戈利木单抗注射液
商品名称:欣普尼 Simponi
® ®
英文名称:Golimumab Injection
汉语拼音:Gelimu Dankang Zhusheye
【成份】
主要成份:戈利木单抗
辅料:山梨醇、L-组氨酸、L-组氨酸盐酸盐一水合物、聚山梨酯 80、注射用水
【性状】
应为无色至淡黄色液体。
【适应症】
类风湿关节炎
本品联合甲氨蝶呤( MTX)适用于治疗对包括 MTX在内的改善病情抗风湿药物
(DMARDs)疗效不佳的中到重度活动性类风湿关节炎成年患者。
本品联合 MTX已被证实能够降低关节损害(通过 X线检测)进展的发生率并改善
身体机能。
强直性脊柱炎
本品适用于治疗活动性强直性脊柱炎成年患者。
【规格】
50 mg/0.5 ml/支
【用法用量】
类风湿关节炎
本品 50 mg,每月 1次给药。皮下注射。
本品应与 MTX联合使用。
强直性脊柱炎
本品 50 mg,每月 1次给药。皮下注射。
现有数据表明类风湿关节炎和强直性脊柱炎患者通常在治疗 12-14周内(3-4次给
药后)获得临床应答。在该时间段内未出现治疗获益证据的患者应重新考虑是否继续该
治疗。
漏用剂量
如果患者在计划的给药日忘记注射本品,那么此次漏用药物应当在患者想起的时候
立即注射。应告知患者不要注射双倍剂量来弥补此次漏用药物。
![]()
![]()
![]()
下一次给药应当根据下列要求进行:
•
•
如 2周内发现漏用剂量,则补注射后继续原有注射计划。
如 2周后发现漏用剂量,则补注射后自注射之日起重新建立新注射计划。
使用说明
本品应在医疗服务提供者的指导和监督下使用。如医生许可,患者在接受正确的皮
下注射技术培训后,可以自行注射给药,并按照说明中提供的方法使用本品。指导患者
遵照以下说明给药:
•
为确保正确使用本品,需在注射前 30分钟将预充式注射器从包装盒内取出,并置
于室温下。请勿以任何其他方式对本品进行加热。
•
在给药前,应通过检查窗肉眼检查溶液中有无颗粒及变色的情况。本品为无色至淡
黄色的澄清至略呈乳白色的液体。如果本品出现溶液变色或浑浊,以及异物颗粒,
请勿使用。
•
•
预充式注射器内剩余的任何药品均不得使用。
预充式注射器的针头保护帽含有干燥的天然橡胶(一种乳胶衍生物),应告知对乳
胶过敏的患者不要接触针头保护帽。
•
•
•
给药时,如果需要多次注射,应在身体的不同部位进行注射。
应轮换注射部位进行注射,不得在皮肤触痛、挫伤、发红或硬结的部位进行注射。
任何未使用的药品或废弃材料应按照本地要求进行处置。
【不良反应】
以下不良反应数据主要来自国外进行的临床研究和上市后监测数据。
安全性特征总结
类风湿关节炎、强直性脊柱炎及其他成年患者人群关键性研究的对照阶段中,最常
见的药物不良反应为上呼吸道感染。12.6%本品治疗组患者和 11.0%对照组患者出现上呼
吸道感染。本品报告的最严重的药物不良反应包括严重感染(包括脓毒症、感染性肺炎、
结核病、侵袭性真菌感染和机会感染)、脱髓鞘类疾病、乙型肝炎再激活、充血性心力
衰竭、自身免疫性疾病(类狼疮综合征)、血液学反应、严重全身性超敏反应(包括速
发过敏反应)、血管炎、淋巴肿瘤和白血病(见【注意事项】)。
不良反应列表
表 1中列出了在临床研究中观察到的药物不良反应以及本品上市后全世界报告的
药物不良反应。药物不良反应按系统器官分类和频率分类,标准如下:十分常见(≥1/10);
常见(≥1/100,<1/10);偶见(≥1/1,000,<1/100);罕见(≥1/10,000,<1/1,000);
十分罕见(<1/10,000);未知(无法从现有数据估算)。在每个频率组中,不良反应按
严重程度从高到低的顺序排列。
![]()
![]()
表 1 药物不良反应列表
感染及侵染类疾病
十分常见:上呼吸道感染(鼻咽炎,咽炎,喉炎和鼻炎)
常见:细菌性感染(如蜂窝织炎),下呼吸道感染(如感染性肺
炎),病毒感染(如流行性感冒和疱疹),支气管炎,鼻窦
炎,浅表真菌感染,脓肿
偶见:脓毒症(包括脓毒性休克),肾盂肾炎
罕见:结核病,机会感染[如侵袭性真菌感染(组织胞浆菌病,
球孢子菌病,肺孢子菌病),细菌性,非典型分枝杆菌感
染和原虫],乙型肝炎复发,细菌性关节炎,感染性滑囊
炎
良性、恶性及性质不明的肿瘤
偶见:肿瘤(如皮肤癌,鳞状细胞癌和黑色素痣)
罕见:淋巴肿瘤,白血病,黑素瘤,Merkel细胞癌
未知:肝脾 T 细胞淋巴瘤*,儿科恶性肿瘤
血液及淋巴系统疾病
常见:白细胞减少症(包括中性粒细胞减少症),贫血
偶见:血小板减少症,全血细胞减少症
罕见:再生障碍性贫血
各类检查
偶见:嗜中性粒细胞计数降低
免疫系统疾病
常见:过敏反应(支气管痉挛,超敏反应,荨麻疹),自身抗体
阳性
罕见:严重全身性超敏反应(包括速发过敏反应),血管炎(系
统性),结节病
内分泌系统疾病
偶见:甲状腺疾病(如甲状腺功能减退症,甲状腺功能亢进症和
甲状腺肿)
代谢及营养类疾病
精神病类
偶见:血葡萄糖升高,脂质升高
常见:抑郁,失眠
各类神经系统疾病
常见:头晕,头痛,感觉错乱
偶见:平衡障碍
罕见:脱髓鞘类疾病(中央及外周),味觉障碍
眼器官疾病
偶见:视力障碍(如视物模糊和视觉灵敏度下降),结膜炎,眼
过敏症(如瘙痒和刺激)
心脏器官疾病
偶见:心律不齐,缺血性冠状动脉疾病
罕见:充血性心力衰竭(新发或恶化)
血管与淋巴管类疾病
常见:高血压
偶见:血栓症(如深静脉和主动脉),潮红
罕见:雷诺现象
呼吸系统、胸及纵隔疾病
胃肠系统疾病
常见:哮喘和相关症状(如哮鸣和支气管功能亢进)
偶见:间质性肺疾病
常见:消化不良,胃肠和腹痛,恶心,胃肠道炎症疾病(如胃炎
和结肠炎),口腔黏膜炎
偶见:便秘,胃食管反流病
肝胆系统疾病
常见:丙氨酸氨基转移酶升高,天门冬氨酸氨基转移酶升高
偶见:胆石症,肝脏疾病
皮肤及皮下组织类疾病
常见:瘙痒,皮疹,脱发,皮炎
偶见:大疱性皮肤反应,银屑病(新发或既存银屑病恶化,手掌
/足底和脓疱),荨麻疹
罕见:苔藓样反应,皮肤剥脱,血管炎(皮肤)
各种肌肉骨骼及结缔组织疾病
罕见:类狼疮综合征
肾脏及泌尿系统疾病
生殖系统及乳腺疾病
罕见:膀胱疾病,肾脏疾病
偶见:乳腺疾病,月经紊乱
全身性疾病及给药部位各种反应
常见:发热,乏力,注射部位局部反应(如注射部位红斑,荨麻
疹,硬结,疼痛,青肿,瘙痒,刺激和感觉错乱),胸部
不适
罕见:延迟愈合
各类损伤、中毒及手术并发症
常见:骨折
*:在其他 TNF阻滞剂中观察到。
部分药物不良反应的描述
感染
在关键性试验的对照研究阶段, 23.0%的本品治疗患者观察到感染,对照患者为
20.2%。在中位随访期约为 4年的研究中,本品治疗患者每 100患者年的感染发生率为
81.1起事件(95% CI:79.5,82.8)。
在关键性试验的对照研究阶段,上呼吸道感染是最常见的不良反应,本品治疗的患
者中有 12.6%报告该不良反应,对照患者为 11.0%。在中位随访期约为 4年的研究中,
本品治疗患者每 100患者年的上呼吸道感染发生率为 34.9起事件(95% CI:33.8,36.0)。
![]()
在类风湿关节炎、强直性脊柱炎和其他适应症试验的对照研究期间,1.2%本品治疗
患者和 1.2%对照治疗患者观察到严重感染。在本品治疗患者中观察到的严重感染包括结
核病、细菌性感染(包括脓毒症和感染性肺炎)、侵袭性真菌感染和其他机会感染。这
些感染中有一部分是致命性的。在中位随访时间至多 3年的关键性试验中,与接受本品
50 mg治疗的患者相比,本品 100 mg治疗的患者严重感染(包括机会感染和结核病)
发生率更高。
恶性肿瘤
淋巴肿瘤
在关键性试验中,本品治疗患者淋巴肿瘤的发生率高于普通人群中的预期发生率。
在中位随访时间至多 3年的上述试验中,本品 100 mg治疗的患者中淋巴肿瘤的发生率
较本品 50 mg治疗的患者高。大多数淋巴肿瘤事件发生在入选患者既往暴露于抗 TNF药
物,且病程更长、病情更难治的 GO-AFTER研究中(见【注意事项】)。
淋巴肿瘤以外的恶性肿瘤
在关键性试验的对照研究阶段以及约 4年的随访期间,本品治疗组和对照组间的非
淋巴肿瘤恶性肿瘤发生率(非黑素瘤皮肤癌除外)相似。此外,非淋巴肿瘤恶性肿瘤(非
黑素瘤皮肤癌除外)的发生率也与普通人群中的发生率相似。
脱髓鞘类疾病
在中位随访时间至多 3年的关键试验中,与接受本品 50 mg治疗患者相比,本品
100 mg治疗的患者脱髓鞘的发生率更高(见【注意事项】)。
肝酶升高
在国外类风湿关节炎和另一适应症关键试验的对照研究期间,本品治疗组和对照组
有相似比例的患者(22.1%-27.4%的患者)发生轻度 ALT升高事件(>1且<3×正常上限
(ULN));而在国外强直性脊柱炎和另一适应症关键研究中,本品治疗患者(26.9%)发
生该事件的比例高于对照组患者(10.6%)。在中位随访期约为 5年的类风湿关节炎试验
和另一适应症试验中,本品治疗患者和对照患者中轻度 ALT升高的发生率相似。
在国外类风湿关节炎和强直性脊柱炎关键试验的对照研究阶段,与对照组(0.0%)
相比本品治疗组中 ALT升高≥5×ULN的事件发生率为 0.4%-0.9%。在中位随访期为 5年
的类风湿关节炎、强直性脊柱炎和另一适应症试验中,本品治疗患者和对照患者中 ALT
升高≥5×ULN的发生率相似。一般而言,这些 ALT升高是无症状的,在继续使用本品、
停用本品或调整合并用药后,该异常现象减轻或消退。
在国外类风湿关节炎试验中 1例既存肝功能异常患者使用了本品及其他可能影响
肝功能的药物,出现了致命的伴有黄疸的非传染性肝炎。不排除本品在此过程中发挥了
作用或使病情恶化。
注射部位反应
![]()
![]()
在关键试验的对照研究阶段,本品治疗患者和对照治疗患者分别有 5.4%和 2.0%出
现注射部位反应。大多数注射部位反应表现为轻度和中度,最常见的表现是注射部位红
斑。注射部位反应未必导致停药。戈利木单抗抗体的存在可能会增加注射部位反应的风
险。
在治疗类风湿关节炎、强直性脊柱炎和其他成年患者的 II/III期试验对照期内,没有
患者出现被认为与本品相关的速发过敏反应。
自身免疫抗体
在关键试验的对照期及至 1年的随访中,本品治疗患者和对照患者中分别有 3.5%
和 2.3%为新发抗核抗体(ANA)阳性(滴度≥1:160)。在 1年随访时,基线抗 dsDNA阴
性的患者产生抗 dsDNA抗体的比例为 1.1%。
【禁忌】
对活性成分或任何辅料(列于【成份】)存在超敏反应。
活动性结核病或其他重度感染例如脓毒症和机会感染(见【注意事项】)。
中度或重度心力衰竭(NYHA III/IV级)(见【注意事项】)。
【注意事项】
感染
在接受本品治疗前、治疗期间和治疗结束后,必须密切监测感染(包括结核病)。
因为本品的清除可能需要长达 5个月的时间,所以在此期间应继续监测患者。如果患者
出现严重感染或脓毒症,则不得再次使用本品(见【禁忌】)。
本品不应用于有临床严重、活动性感染的患者。在考虑给有慢性感染或有复发性感
染病史的患者使用本品时,应谨慎。应告知患者感染的潜在危险因素,并应避免暴露于
感染的潜在危险因素中。
使用 TNF阻滞剂的患者更容易发生严重感染。
在接受本品治疗的患者中曾经报告了细菌性感染(包括脓毒症和感染性肺炎)、分
枝杆菌感染(包括结核病)、侵袭性真菌感染和机会感染,包括可导致死亡的感染。患
者经常表现为播散性而非局灶性感染。合并免疫抑制治疗的患者曾发生过部分上述严重
感染,加之这些患者常常伴有基础疾病,使他们更易发生感染。应密切监测在接受本品
治疗时出现新发感染的患者,并对患者进行全面的诊断评估。如果患者发生新的严重感
染或脓毒症,应停用本品并开始采用适当的抗微生物治疗或抗真菌治疗,直至感染得到
控制。
对于居住或曾前往侵袭性真菌感染(例如:组织胞浆菌病、球孢子菌病或芽生菌病)
流行地区的患者,在开始或继续本品治疗之前应谨慎考虑本品治疗的获益和风险。在接
受本品治疗的有感染风险的患者中,如果其出现严重全身性疾病则应怀疑侵袭性真菌感
染。在一些活动性感染患者中,抗原和抗体检测可能阴性。如可行,对该类患者进行诊
![]()
断以及给予经验性抗真菌治疗时,应咨询侵袭性真菌感染专科医生,并且应该同时考虑
重度真菌感染的风险和抗真菌治疗的风险。
结核病
在接受本品治疗的患者中已有结核病报告。在大多数报告中,结核病是肺外结核,
表现为局灶性或播散性。
在开始本品治疗前,应对所有患者进行活动性结核和非活动性(“潜伏性”)结核的
评估。本评估应包括详细病史:结核病个人史,既往可能的结核病接触史,既往和 /或
现在免疫抑制治疗情况。应对所有患者进行适当的筛查(即结核菌素皮肤试验或血液检
查和胸部 X线检查)或可遵照当地建议。处方者应注意结核菌素皮肤试验结果假阴性的
风险,特别是在重症或免疫受损的患者中。
如果诊断有活动性结核病,不得开始本品治疗(见【禁忌】)。
如果怀疑潜伏性结核,应向结核病治疗专科医生咨询。在所有下述情况下,应慎重
权衡本品治疗的获益与风险。
如果诊断有非活动性(“潜伏性”)结核,在开始本品治疗前须开始抗结核病治疗,
并遵照当地的建议。
对于潜伏性结核检查结果呈阴性但有几种或有显著的结核病危险因素的患者,在开
始本品治疗前应考虑进行抗结核病治疗。只有在咨询结核病治疗专科医生并考虑潜伏结
核感染风险和抗结核病治疗风险后,方可做出对上述患者是否开始抗结核病治疗的决
定。对有潜伏性结核或活动性结核病既往病史且不能确定已接受足够疗程治疗的患者,
在开始本品治疗前也应考虑进行抗结核病治疗。
接受本品治疗的患者在潜伏性结核治疗过程中及结束后,曾报告出现过活动性结核
病。对接受本品治疗的患者(包括潜伏性结核检查结果阴性的患者、接受潜伏性结核治
疗的患者,及既往接受过结核感染治疗的患者),应密切监测活动性结核病的症状和体
征。
应告知所有患者,在本品治疗期间或治疗结束后,如果出现提示结核病的体征 /症
状(如持续性咳嗽、消耗/体重下降、低热),应就医。
乙型肝炎再激活
在接受包括本品在内的 TNF阻滞剂治疗的乙型肝炎病毒慢性携带者(即表面抗原阳
性者)中,已有出现乙型肝炎复发,有些病例发生了致死性的结局。
本品治疗前应对患者进行 HBV感染检查。对于 HBV感染检查阳性的患者,建议咨
询乙肝治疗专科医生。
对于需要接受本品治疗的 HBV携带者,应在治疗开始前、治疗的过程中及治疗结束
后的几个月内,适当评估和密切监测活动性 HBV感染的症状和体征。对于 HBV携带者
合并预防 HBV再激活抗病毒治疗和 TNF阻滞剂治疗的数据尚不足。出现 HBV再激活的
患者应停用本品,并开始使用有效的抗病毒治疗和适当的支持疗法。
![]()
恶性肿瘤和淋巴组织增殖性疾病
尚不明确 TNF阻滞剂治疗在恶性肿瘤发生中的潜在作用。根据当前的认知,不能排
除接受 TNF阻滞剂治疗的患者发生淋巴肿瘤、白血病或其他恶性肿瘤的可能的风险。在
考虑对有恶性肿瘤病史的患者进行 TNF阻滞疗法或考虑对发生恶性肿瘤的患者继续使
用 TNF阻滞剂治疗时,应谨慎。
儿科恶性肿瘤
本品在中国尚未批准用于儿童和青少年患者,但国外相关研究数据和上市后资料中
提示儿童和青少年患者使用包括本品在内的 TNF阻滞剂治疗时,已报告有淋巴肿瘤和其
他恶性肿瘤发生(部分为致死性)。
上市后,在使用 TNF阻滞剂治疗(开始接受治疗时的年龄≤18岁)的儿童、青少
年和年轻成人(直至 22岁)中,已有恶性肿瘤病例报告,其中有些是致死性的。大约
有一半病例为淋巴肿瘤,其他的病例表现为多种不同的恶性肿瘤,包括通常与免疫抑制
相关的罕见恶性肿瘤。不能排除接受 TNF阻滞剂治疗的儿童和青少年发生恶性肿瘤的风
险。
淋巴肿瘤和白血病
所有 TNF阻滞剂(包括本品)的临床试验对照研究阶段,抗 TNF治疗组观察到的淋
巴肿瘤病例多于对照组。在类风湿关节炎和强直性脊柱炎等的 IIb期和 III期临床试验期
间,本品治疗患者的淋巴肿瘤发生率高于普通人群的预期值。接受本品治疗的患者曾报
告白血病。长期炎症活动的类风湿关节炎患者较一般人群发生淋巴肿瘤及白血病的危险
性增加。
在接受其他 TNF阻滞剂治疗的患者中,曾报告过罕见的肝脾 T细胞淋巴瘤(HSTCL)
的上市后病例(见【不良反应】)。该类罕见 T细胞淋巴肿瘤侵袭性强,常导致死亡。大
多病例发生于青少年和年轻成年男性,且几乎所有病例均为接受过硫唑嘌呤(AZA)或
6-巯基嘌呤(6-MP)与 TNF阻滞剂联合治疗的炎症性肠病患者。应慎重考量 AZA或 6-MP
和本品联用的潜在风险。不能排除接受 TNF阻滞剂治疗的患者发生肝脾 T细胞淋巴瘤的
风险。
淋巴肿瘤以外的其他恶性肿瘤
在类风湿关节炎、强直性脊柱炎及其他成年患者人群 IIb期和 III期临床试验对照研
究阶段,本品治疗组非淋巴肿瘤恶性肿瘤(非黑素瘤皮肤癌除外)的发生率和对照组相
似。
结肠异型增生/结肠癌
目前尚不清楚本品治疗是否会影响结肠异型增生或结肠癌的风险。对于结肠异型增
生或结肠癌风险增加(例如,患有长期溃疡性结肠炎或原发性硬化性胆管炎的患者),
或者有结肠异型增生或结肠癌病史的溃疡性结肠炎患者,在治疗前和在整个病程中应定
期进行筛查。具体的评价包括按照当地建议进行结肠镜检查和活检。对新诊断结肠异型
![]()
增生的接受本品治疗的患者,必须慎重权衡个体患者的风险和获益,考量是否继续治疗。
皮肤癌
接受 TNF阻滞剂(包括本品)治疗的患者曾报告黑素瘤和 Merkel细胞癌(见【不
良反应】)。建议定期进行皮肤检查(特别是对于有皮肤癌风险因素的患者)。
充血性心力衰竭
TNF阻滞剂(包括本品)治疗时曾报告充血性心力衰竭恶化和新发的病例,某些病
例具有致死性结局。在使用另一种 TNF阻滞剂的一项临床试验中,曾观察到充血性心力
衰竭恶化以及由充血性心力衰竭引起的死亡率增加。尚未在充血性心力衰竭患者中进行
本品的研究。轻度心力衰竭(NYHA分级 I/II级)患者应慎用本品。应对患者进行密切监
测,一旦出现新的心力衰竭症状或心力衰竭的症状恶化,则必须停用本品(见【禁忌】)。
脱髓鞘疾病
TNF阻滞剂(包括本品)的使用,与中枢神经系统脱髓鞘疾病(包括多发性硬化症)
及外周脱髓鞘疾病(包括 Guillain-Barré综合征)的临床症状和/或放射学证据新发或加重
的病例有关。对于既存或新近发生脱髓鞘疾病的患者,应在开始本品治疗前慎重考虑抗
TNF治疗的获益和风险。如果患者发生上述脱髓鞘疾病应考虑停用本品(见【不良反应】)。
手术
曾经接受过手术(包括关节成形术)的患者接受本品治疗的安全性经验有限。如果
计划进行手术,应考虑到本品的半衰期较长。对需要手术的本品使用者应密切监测感染,
并采取适当的措施。
免疫抑制
由于 TNF起介导炎症和调节细胞免疫应答的作用,因此 TNF阻滞剂(包括本品)可
能会影响宿主抵御感染和恶性肿瘤的能力。
自身免疫
使用 TNF阻滞剂(包括本品)可导致抗核抗体(ANA)产生,罕见导致类狼疮综合
征的发生(见【不良反应】)。如果患者在接受本品治疗后出现提示类狼疮综合征的症状
应停用本品。
血液学反应
在使用 TNF阻滞剂的患者中,已有全血细胞减少症、白细胞减少症、中性粒细胞减
少症、粒细胞缺乏症、再生障碍贫血和血小板减少症的上市后报告。在本品临床试验中
已有报告血细胞减少(包括全血细胞减少症)。对当前或既往有显著血细胞减少的患者
使用本品应谨慎。应告知所有患者如果出现提示血液系统疾病的症状和体征(如持续性
发热、青肿、出血和苍白),应立即就医。对有确定的显著血液系统异常的患者,应考
虑停用本品。
![]()
![]()
![]()
![]()
![]()
![]()
与阿那白滞素联用
在阿那白滞素与另一种 TNF阻滞剂依那西普联用的临床研究中,曾观察到严重感染
和中性粒细胞减少症,并未显示额外的临床获益。根据阿那白滞素与依那西普联用发现
的不良事件的性质,推测阿那白滞素与其他 TNF阻滞剂联用也可能导致类似的毒性。不
建议本品与阿那白滞素联用。
与阿巴西普联用
与单用 TNF阻滞剂相比,在临床研究中,TNF阻滞剂与阿巴西普联用观察到与其有
关的感染(包括严重感染)风险的增加,而临床获益并未增加。不建议本品与阿巴西普
联用。
与其他生物疗法联用
有关本品与治疗本品相同适应症的其他生物疗法联用的信息并不充分。由于本品与
这些生物制剂联用可能会增加感染风险,并且可能会发生其他潜在的药理学相互作用,
因此不建议联用。
生物制剂类 DMARDs间的转换使用
当从一种生物制剂转换为另一种生物制剂时,由于其生物活性重叠可能会进一步增
加不良事件(包括感染)的风险,因此,当转换使用时应注意并应继续对患者进行监测。
疫苗接种/治疗用感染性制剂
接受本品治疗的患者可合并疫苗接种,但活疫苗除外(见【药物相互作用】和【孕
妇及哺乳期妇女用药】)。在接受抗 TNF治疗的患者中,关于对活疫苗接种的反应以及活
疫苗引发的继发感染传播的数据较为有限。使用活疫苗能导致临床感染,包括播散性感
染。
在一项国外其他适应症 III期研究中接受本品治疗的患者对肺炎球菌多糖疫苗可产
生有效 B细胞免疫应答。相似数量的接受本品和未接受本品治疗的患者抗体滴度有至少
2倍的增加。与未接受 MTX的患者相比,接受 MTX的本品治疗组和对照治疗组中对肺
炎球菌疫苗应答的患者的比例更低。
使用治疗用感染性制剂导致临床感染,包括播散性感染。建议本品不与治疗用感染
性制剂联用。
过敏反应
在接受本品治疗的患者中,已有严重全身超敏反应(包括速发过敏反应)的上市后
报告。其中有一些严重全身超敏反应发生在首次使用本品后。如果发生速发过敏反应或
其他严重过敏反应,应立即停用本品,并开始适当的治疗。
![]()
![]()
![]()
![]()
![]()
![]()
乳胶过敏
预充式注射器的针头保护帽是由含有乳胶的干燥天然橡胶制造的,对于乳胶过敏的
人可能会引起过敏反应。
肾及肝损害
肝功能受损的患者应慎用本品。尚未在伴有肾或肝损害的患者中进行本品的特定研
究。无法提供剂量建议。
辅料
本品包含山梨醇,遗传性果糖不耐受患者不应使用本品。
对驾驶和操作机器能力的影响
本品可能对驾驶和操作机器的能力有轻微的影响。使用本品后可能会出现头晕(见
【不良反应】)。
【孕妇及哺乳期妇女用药】
有生育能力的女性
育龄妇女必须采取适当的避孕措施以防止怀孕,并且在末次本品治疗后持续采取避
孕措施至少 6个月。
妊娠
本品在孕妇中使用的数据不足。由于本品抑制 TNF,在妊娠期间使用本品可影响新
生儿正常的免疫应答。动物研究未显示对于妊娠、胚胎 /胎儿发育、分娩或出生后发育
直接或间接的有害影响(见【毒理研究】)。不建议孕妇使用本品,只有在确实需要时方
可给予本品。
本品可穿过胎盘。自孕妇在妊娠期间接受 TNF阻滞剂单克隆抗体治疗起至该女性产
下婴儿 6个月,均可在婴儿的血清中检测到抗体。因此,这些婴儿的感染风险可能增高。
如果孕妇在妊娠期间接受本品注射,对于曾在宫内暴露于本品的婴儿,不建议在母亲妊
娠期间末次本品注射后 6个月内给予活疫苗(见【注意事项】和【药物相互作用】)。
哺乳
尚不明确本品给药后,是否会在人乳汁中分泌或是否全身吸收。已证明本品经猴的
母乳排出,又因人免疫球蛋白可经母乳排出,所以女性在本品治疗期间及治疗后至少 6
个月内不得哺乳。
生育力
尚未使用本品进行动物生育力研究。在一项小鼠生育力研究中,使用了一种选择性
抑制小鼠 TNFa功能活性的类似抗体,未观察到其对生育力的相关影响(见【毒理研究】)。
![]()
![]()
![]()
![]()
![]()
![]()
![]()
【儿童用药】
目前尚没有本品在中国儿童患者中进行的安全性和有效性研究的资料,故不推荐使
用。
【老年用药】
老年患者(≥65岁)
老年患者不需要剂量调整。
在类风湿关节炎、强直性脊柱炎和其他适应症 III期研究中,接受本品治疗的 65岁
或以上患者与年轻患者相比,在不良事件、严重不良事件和严重感染方面未观察到总体
差异。然而,在治疗老年患者时应谨慎,并应特别注意有无感染发生。
【药物相互作用】
甲氨蝶呤
未观察到甲氨喋呤对本品静脉给药的清除率产生显著影响。虽然皮下注射给药时,
与甲氨蝶呤联用导致本品在类风湿关节炎、强直性脊柱炎或另一风湿病患者中的稳态谷
浓度升高,但是此数据并未表明需要调整本品或甲氨蝶呤的剂量(见【药代动力学】)。
与其他生物疗法联用
不建议本品与治疗本品相同适应症的其他生物疗法(包括阿那白滞素和阿巴西普)
联用(见【注意事项】)。
活疫苗/治疗用感染性制剂
本品不应与活疫苗联用(见【注意事项】和【孕妇及哺乳期妇女用药】)。
本品不应与治疗用感染性制剂联用(见【注意事项】)。
细胞色素 P450底物
慢性炎症期间细胞因子(例如 TNFa)水平升高可能抑制 CYP450酶的生成。因此,
对于拮抗细胞因子活性的分子(例如本品)而言,预期 CYP450酶的生成可恢复正常。
接受治疗指数较窄的 CYP450底物治疗的患者在本品开始用药或停药时,建议监测药效
(例如华法林)或药物浓度(例如环孢霉素或茶碱),并且根据需要调整个体药物剂量。
【药物过量】
在一项临床试验中,有 5例患者接受试验方案指定的本品给药剂量(10 mg/kg)单
次静脉输注,未出现严重不良反应或其他有意义的反应。患者的最高体重为 100 kg,因
此单次静脉输注本品 1000 mg。
如出现用药过量,建议监测不良效应的任何体征或症状,立即采取适当的对症治疗。
![]()
![]()
![]()
![]()
【临床试验】
以下为本品在国外进行的临床研究。
类风湿关节炎
三项多中心、随机、双盲、安慰剂对照研究证明了本品的有效性。研究共纳入 1500
余例年龄≥18岁,根据美国风湿病学会(ACR)的标准在筛选时被确诊为中到重度活动
性类风湿关节炎至少 3个月,且至少伴有 4个关节肿胀和 4个关节压痛的患者。每隔 4
周经皮下注射一次本品或安慰剂。
GO-FORWARD研究中评价了 444例患者,这些患者在接受了至少为 15 mg/周的固
定剂量的 MTX治疗后类风湿关节炎仍有活动,并且从未接受过抗 TNF药物治疗。患者
随机接受安慰剂+MTX、本品 50 mg+MTX、本品 100 mg+MTX或本品 100 mg+安慰剂治
疗。第 24周后,接受安慰剂+MTX治疗的患者改为接受本品 50 mg+MTX治疗。第 52
周时,患者进入开放性长期扩展研究。
GO-AFTER研究中评价了既往接受过 1种或多种抗 TNF药物(阿达木单抗、依那西
普或英夫利西单抗)治疗的 445例患者。患者终止既往抗 TNF治疗的原因主要是缺乏有
效性(58%)、不能耐受(13%)和/或安全性或有效性之外的原因(29%,主要是出于经
济原因)。患者随机接受安慰剂、本品 50 mg或本品 100 mg。允许患者在研究期间继
续合并使用 MTX、柳氮磺胺吡啶(SSZ)和/或羟基氯喹(HCQ)等 DMARD治疗。
GO-BEFORE研究中评价了既往未接受过 MTX治疗及抗 TNF药物治疗的 637例活动
性类风湿关节炎患者。患者随机接受安慰剂+MTX、本品 50 mg+MTX、本品 100 mg+MTX
或本品 100 mg+安慰剂治疗。第 52周时,患者进入开放的长期扩展研究。在长期扩展
研究中,接受安慰剂+MTX治疗的患者出现至少 1处关节压痛或关节肿胀改为接受本品
50 mg+MTX治疗。
GO-FORWARD研究中的主要(共同主要)终点是:在第 14周达到 ACR 20应答的患
者百分比,以及第 24周时的健康评定问卷(HAQ)评分相对于基线水平的改善。GO-AFTER
研究中的主要终点是在第 14周达到 ACR 20应答的患者百分比。GO-BEFORE研究中的共
同主要终点是在第 24周达到 ACR 50应答的患者百分比以及第 52周时的 van der Heijde-
modified Sharp(vdH-S)评分相对于基线水平的改变。除了主要终点外,还评价了本品治疗
对关节炎症状和体征、影像学应答、身体机能及健康相关生活质量的影响。
总体来说,研究 GO-FORWARD和 GO-BEFORE至第 104周以及研究 GO-AFTER至第
24周,比较本品 50 mg和 100 mg合并使用 MTX的疗效时,未见具有临床意义的差异。
症状和体征
表 2中显示了 GO-FORWARD、GO-AFTER和 GO-BEFORE研究中本品 50 mg组在第 14、
24和 52周的主要 ACR结果,描述如下。在开始本品给药后的首次评价(第 4周)时即
观察到疗效。
在 GO-FORWARD研究中,有 89例患者随机进入本品 50 mg+MTX组,其中有 48例
![]()
患者在第 104周继续维持该治疗。在这 48例患者中,有 40例患者在第 104周达到 ACR
20应答,有 33例患者在第 104周达到 ACR 50应答,有 24例患者在第 104周达到 ACR 70
应答。在继续参加研究并接受本品治疗的患者中,从第 104周至第 256周期间观察到
ACR 20、ACR 50或 ACR 70应答的百分比相似。
在 GO-AFTER研究中,本品治疗组达到 ACR 20应答的患者百分比高于安慰剂组(不
考虑停用既往 1种或多种抗 TNF治疗的原因)。
表 2 GO-FORWARD、GO-AFTER和 GO-BEFORE对照部分的主要疗效结果
GO-FORWARD
活动性类风湿关节炎,
MTX经治
GO-AFTER
活动性类风湿关节炎,既
往接受 1种或多种抗 TNF
药物
GO-BEFORE
活动性类风湿关节炎,
MTX初治
安慰剂
+
MTX
本品
50 mg
+
安慰剂
本品
安慰剂
+
MTX
本品
50 mg
+
50 mg
MTX
89
MTX
159
n
a
133
150
147
160
应答者,占患者的%
ACR 20
第 14周
第 24周
第 52周
33%
28%
NA
55%*
60%*
NA
18%
16%
NA
35%*
31% p = 0.002
NA
NA
49%
52%
NA
62%
60%
ACR 50
第 14周
第 24周
第 52周
10%
14%
NA
35%*
37%*
NA
7%
4%
NA
15% p = 0.021
16%*
NA
NA
29%
36%
NA
40%
42%
ACR 70
第 14周
4%
14%
p = 0.008
20%*
2%
10%
p = 0.005
9% p = 0.009
NA
NA
NA
第 24周
第 52周
5%
NA
2%
NA
16%
22%
24%
28%
NA
a
n代表随机分组患者;在不同时间点每个终点的实际可评价患者例数可能有所不同。
* p ≤ 0.001
NA:不适用
在 GO-BEFORE研究中,第 24周时在中重度类风湿关节炎患者(本品 50 mg+MTX
联合组、本品 100 mg+MTX联合组与 MTX单用组达到 ACR 50应答的患者)中进行的主
要分析结果无统计学意义(p=0.053)。第 52周时,在总体人群中,本品 50 mg+MTX组达
到 ACR应答的患者百分比总体上高于 MTX单用组,但两组之间不存在显著差异(见表
2)。在有重度、活动性和进展性类风湿关节炎的亚组人群中进行了额外的分析。与在总
体人群中相比,在该特定人群中本品 50 mg+MTX效果总体上优于 MTX单用。
在 GO-FORWARD和 GO-AFTER研究中,在每个预设的时间点、第 14周和第 24周观
察到的疾病活动性量表(DAS)应答具有临床意义和统计学意义( p≤0.001)。在研究开
始时随机进入本品组且仍然继续本品治疗的患者可一直维持 DAS28应答水平至第 104
周。在继续参加研究并接受本品治疗的患者中,从第 104周至第 256周期间观察到 DAS28
应答率相似。
在 GO-BEFORE研究中,评估了主要临床应答(定义为连续 6个月以上维持 ACR 70
应答)。第 52周时,本品 50 mg+MTX组有 15%的患者达到主要临床应答,而安慰剂+MTX
组则有 7%的患者达到主要临床应答(p=0.018)。在随机进入本品 50 mg+MTX组的 159
例患者中,有 96例患者在第 104周时仍然继续该治疗。其中分别有 85、66和 53例患
者在第 104周获得 ACR 20/50/70应答。在继续参加研究并接受本品治疗的患者中,从第
104周至第 256周期间观察到 ACR 20、ACR 50或 ACR 70应答率相似。
影像学应答
在 GO-BEFORE研究中,vdH-S评分相对于基线水平的改善被用于评价结构破坏的程
度。该评分是通过影像学检查测量手 /腕部和足部关节侵蚀数量及程度,和关节腔狭窄
程度等结构破坏的一种综合分数。表 3中列出了本品 50 mg剂量组在第 52周时的主要
结果。
在无新生关节侵蚀或 vdH-S总评分相对于基线水平的改变≤0的患者数量方面,本品
治疗组显著高于对照组(p=0.003)。在 104周期间,一直可维持第 52周观察到的影像
学应答。在继续参加研究并接受本品治疗的患者中,从第 104周至第 256周期间影像学
应答相似。
表 3在 GO-BEFORE研究的总体人群中,第 52周时 vdH-S总评分相对于基线水平的影像
学平均(SD)改变
安慰剂+MTX
本品 50 mg+MTX
n
a
160
159
总分
基线
19.7 (35.4)
1.4 (4.6)
18.7 (32.4)
0.7 (5.2)
*
相对于基线的变化
侵蚀评分
基线
11.3 (18.6)
0.7 (2.8)
10.8 (17.4)
0.5 (2.1)
相对于基线的变化
JSN评分
基线
8.4 (17.8)
0.6 (2.3)
7.9 (16.1)
0.2 (2.0)
**
相对于基线的变化
a
n表示随机分组的患者
* p = 0.015
** p = 0.044
身体机能和健康相关生活质量
在 GO-FORWARD和 GO-AFTER研究中,使用 HAQ DI残疾指数分别评价身体机能和
![]()
![]()
残疾这两个终点。在这些研究中,第 24周的 HAQ DI评分相对于基线的改善,本品与对
照药物之间的差异具有临床意义和统计学意义。对于在研究开始时随机进入本品组且仍
继续本品治疗的患者,他们在 104周期间一直维持 HAQ DI改善。在继续参加研究并接
受本品治疗的患者中,从第 104周至第 256周期间观察到 HAQ DI改善的百分比相似。
在 GO-FORWARD研究中,第 24周时使用 SF-36评分中的身体机能各组分评分,来
测量健康相关生活质量。本品治疗患者与安慰剂相比,观察到的健康相关生活质量评分
的改善,具有临床意义和统计学意义。对于在研究开始时随机进入本品组且仍然继续本
品治疗患者,他们在 104周期间一直维持 SF-36身体机能各组分评分的改善。在继续参
加研究并接受本品治疗的患者中,从第 104周至第 256周期间 SF-36身体机能各组分评
分的改善相似。在 GO-FORWARD研究和 GO-AFTER研究中,使用慢性病功能评价-疲劳
量表(FACIT-F)测量疲劳,观察到疲劳评分的改善具有统计学意义。
强直性脊柱炎
在一项多中心、随机、双盲、安慰剂对照研究(GO-RAISE研究)中,对本品的安全
性和有效性进行了评价。该研究共纳入了 356例活动性强直性脊柱炎成年患者[定义为:
在 0~10cm的量表上,Bath强直性脊柱炎活动性指数(BASDAI)≥4且脊柱痛 VAS评分≥4]。
纳入本研究的患者是正在接受或曾接受过 NSAID或 DMARD治疗,并且先前未接受过抗
TNF治疗的活动性疾病患者。患者每隔 4周经皮下注射一次本品或安慰剂。患者被随机
分至安慰剂组、本品 50 mg组或本品 100 mg组,并允许其继续接受 DMARD伴随治疗
(MTX、SSZ和/或 HCQ)。主要终点是第 14周时达到强直性脊柱炎疗效评价标准(ASAS)
20应答的患者百分比。至第 24周,收集并分析安慰剂对照期的疗效数据。
总的来说至第 24周,在对本品 50 mg和本品 100 mg两种给药方案的疗效评价中,
未观察到两者间具有临床意义的差异。表 4列出了本品 50 mg剂量组的主要结果,描
述如下。
表 4 GO-RAISE研究中的主要疗效结果
本品
安慰剂
50 mg*
138
n
a
78
应答者,占患者的%
ASAS 20
第 14周
第 24周
22%
23%
59%
56%
ASAS 40
ASAS 5/6
第 14周
第 24周
15%
15%
45%
44%
第 14周
第 24周
8%
13%
50%
49%
* 所有比较 p ≤ 0.001
n代表随机分组患者;在不同时间点每个终点的实际可评价患者例数可能有
所不同。
a
在继续参加研究并接受本品治疗的患者中,从第 24周至第 256周期间获得 ASAS 20
和 ASAS 40应答的患者比例相似。
在第 14周和第 24周观察到的 BASDAI 50、BASDAI 70和 BASDAI 90评分应答也具有
统计学意义(p≤0.017)。在开始本品治疗后的首次评价(第 4周)时,观察到主要的疾
病活动性指标有所改善,并且一直维持到第 24周。在继续参加研究并接受本品治疗的
患者中,从第 24周至第 256周期间观察到 BASDAI较基线变化的百分比相似。无论患者
是否合并使用 DMARD(MTX、SSZ和/或 HCQ),以及 HLA-B27抗原检测结果或基线 CRP
水平,患者第 14周时的 ASAS 20应答均能获得持续疗效。
在第 14周和第 24周使用 BASFI相对于基线水平的改变评价身体机能,结果表明本
品治疗显著改善了身体机能。在第 14周和第 24周使用 SF-36量表评估身体机能以及健
康相关生活质量,也表明本品治疗显著改善了健康相关生活质量。继续研究且接受本品
的患者中,自第 24周至第 256周生理机能和健康相关生活质量改善相似。
免疫原性
类风湿关节炎、强直性脊柱炎和另一适应症 III期研究 52周期间,在 5%(105/2062)
的接受本品治疗的患者中通过酶免疫测定(EIA)法检测到戈利木单抗抗体,几乎所有的抗
体在体外试验中都能够被中和。在各种风湿性疾病患者中观察到的发生率相似。联用
MTX治疗组产生戈利木单抗抗体的患者比例低于未联用 MTX的本品单药治疗组,分别
约为 3%(41/1235)与 8%(64/827)。
戈利木单抗抗体的存在可能会使注射部位反应的发生率升高(见【不良反应】)。由
于戈利木单抗抗体阳性患者的数量较少,目前尚不能确定戈利木单抗抗体与临床有效性
或安全性结果之间的相关性。
免疫原性分析采用针对本药品的特定方法测定,不适于将本品的抗体产生率与其他
药品进行比较。
【药理毒理】
药理作用
戈利木单抗是一种人源化 TNFα单克隆抗体,可与人可溶型和跨模型 TNFα结合,
抑制 TNFα与其受体结合,从而抑制 TNFα的生物学活性。
在体外试验中,戈利木单抗可调节 TNFα介导的生物学效应,包括 TNFα诱导促进
白细胞浸润的粘附因子(E-选择素、VCAM-1、ICAM-1)的表达和促炎细胞因子(IL-6、
IL-8、G-CSF和 GM-CSF)的分泌。
毒理研究
遗传毒性
![]()
尚未开展戈利木单抗的遗传毒性研究。
生殖毒性
在一项采用抗小鼠 TNFα同源抗体进行的小鼠生育力毒性试验中,小鼠生育力未见
明显损伤。
在一项采用食蟹猴进行的胚胎 -胎仔发育毒性试验中,食蟹猴于妊娠第一孕期每周
两次皮下注射给予戈利木单抗达 50mg/kg/次(约为最大推荐人用剂量(MRHD)的 360
倍),未见明显母体毒性和胎仔毒性。妊娠第 2孕期结束时采集的脐带血样品显示,胎
仔在妊娠期间暴露于戈利木单抗。在这项试验中,宫内暴露戈利木单抗未导致食蟹猴胎
仔发育缺陷。
在一项围产期发育毒性试验中,食蟹猴于妊娠第二、第三孕期以及哺乳期每周两次
皮下注射给予戈利木单抗达 50mg/kg/次(母体和胎仔血药浓度分别约是人最大稳态血药
浓度的 860倍和 310倍),母体和幼仔均未见明显毒性损伤。幼仔从出生至出生后 6个
月,血清中均有戈利木单抗暴露。食蟹猴胎仔在妊娠期和哺乳期暴露于戈利木单抗,未
见明显发育缺陷。
在食蟹猴围产期发育毒性试验中,妊娠期和哺乳期给予戈利木单抗,乳汁中可检测
到戈利木单抗,其浓度约是母体血清浓度的 1/400。
妊娠期间 IgG抗体可通过胎盘转移,母亲妊娠期间给予抗体治疗后出生的婴儿血清
中可检测到 IgG抗体。戈利木单抗也是一种 IgG抗体,目前妊娠期间给予戈利木单抗治
疗后出生的婴儿发生感染的风险可能增加,并长达 6个月之久。因此,母亲在妊娠期间
给药戈利木单抗治疗,末次注射 6个月内,不建议其婴儿接种活疫苗。
致癌性
尚未开展戈利木单抗的致癌性试验。
【药代动力学】
吸收
给予健康受试者和类风湿关节炎患者戈利木单抗单次皮下注射后,达到最大血药浓
度的中位时间(Tmax)为 2-6天。给予健康受试者 50 mg戈利木单抗皮下注射后,产生的最
大血药浓度(Cmax)平均值±标准差为 3.2±1.4 mg/mL。对静脉注射或皮下注射给予本品后
的平均 AUCinf
53%。
值进行交叉研究比较,结果显示,皮下注射本品的绝对生物利用度约为
分布
活动性类风湿关节炎患者在单次静脉注射给予 0.1到 10.0mg/kg的剂量后,平均分
布容积的范围为 58-126 mL/kg。该结果表明本品主要分布在循环系统中,在血管外的分
布有限。
清除
活动性类风湿关节炎患者在接受本品单次静脉注射 0.1到 10.0mg/kg后,平均全身
清除率的估算值为 4.9到 6.7 mL/kg/天。
健康受试者和活动性类风湿关节炎、强直性脊柱炎或另一风湿病患者中的中位终末
半衰期的估算值大约为 2周。
群体药代动力学分析显示,联用非甾体类抗炎药、口服糖皮质激素或柳氮磺胺吡啶
不影响本品的表观清除率。
产生抗戈利木单抗抗体的患者中,本品稳态血清谷浓度通常较低。
线性关系
单次静脉注射 0.1~10.0 mg/kg的剂量后,本品在类风湿关节炎患者中药代动力学特
征近似线性。单次皮下注射 50~400 mg的剂量后,本品在健康受试者中的药代动力学特
征同样近似线性。
体重对药代动力学的影响
随着患者体重增加,本品的表观清除率趋势性升高。
【贮藏】
2~8℃避光保存。
【包装】
预充式注射器(1型玻璃)中装有 0.5 mL溶液,有一个固定的针头(不锈钢),预
充式注射器上带有针头保护帽(橡胶中含有乳胶)。
包装规格:1支/盒
【有效期】
22个月
【执行标准】
进口药品注册标准 JS20100106
【进口药品注册证号】
S20170050
【生产企业】
企业名称:Baxter Pharmaceutical Solutions LLC
生产地址:927 South Curry Pike, Bloomington, IN 47403,美国
国内联系方式
名称:西安杨森制药有限公司
地址:陕西省西安市高新区草堂科技产业基地草堂四路 19号
邮政编码:710304
电话号码:400 888 9988
传真号码:(029)8257 6616
网址:http://www.xian-janssen.com.cn
戈利木单抗注射液预充式注射器的使用和处理方法
使用本品之前请仔细阅读该说明书的全部内容,因为该说明书中包含重要的用药信
息。
自我注射指导
请您仔细阅读,并按照其中步骤操作。在确保您已经了解如何进行准备和注射给药
之前,请勿尝试自行注射。在经过专业医护人员的适当培训之后,可自行完成注射给药。
本说明包括:
1. 准备预充式注射器
2. 选择和准备注射部位
3. 注射本品
4. 注射之后
图 1为预充式注射器的示意图。
柱塞
针头防护激活夹
主体
检查窗
针头保护帽
柱塞头
针头防护翼
标签
针头

图1
1. 准备预充式注射器
握住预充式注射器的主体部分
•
•
•
•
•
切勿手握注射器的柱塞头、柱塞、针头防护翼或针头保护帽。
任何时候都切勿向后拉柱塞。
任何时候都切勿摇晃预充式注射器。
在被要求取下针头保护帽之前,切勿将其从预充式注射器上取下。
切勿碰触针头防护激活夹(如图 1中星号(*)标记部分),以防针头防护装置提
前覆盖针头。
检查预充式注射器的数量
检查预充式注射器,以确保
•
预充式注射器的数量和规格是正确的。
如果您的剂量为 50 mg,您将获得一支本品的 50 mg预充式注射器。
检查有效期(见图 2)
•
•
•
仔细查找位于预充式注射器主体内的检查窗,来检查标签上的有效期(以“EXP”指
示)。
如果通过检查窗无法看到有效期,握住预充式注射器的主体,并旋转针头保护帽
使有效期位于该检查窗上。
您还可以检查打印在纸盒上的有效期。
如果已经超过有效期,请勿使用该预充式注射器。有效期指当月最后一天。发生这
种情况时,请联系您的医生或药剂师来获得帮助。
图2
等待 30分钟,使预充式注射器达到室温
•
为了确保注射得当,将预充式注射器从盒中取出后放置在室温下 30分钟,并远离
儿童。
不要用任何其他方式加热预充式注射器(例如,不要用微波或热水加热)。
在待预充式注射器达到室温期间,切勿取下注射器的针头保护帽。
准备好其余的用品
当您在等待时,可以准备好其余的用品,包括酒精棉签、棉球或纱布和锐器盒。
检查注射器中的液体
•
•
握住预充式注射器的主体部分,且将盖好的针头端朝下。
通过预充式注射器的检查窗检查液体,并确保它为澄清至略带乳光(有珍珠般光
泽)以及无色至淡黄色。该溶液可能含有少许半透明的或白色的蛋白质小颗粒。
如果通过检查窗无法看到液体,握住预充式注射器的主体,并旋转针头保护帽使
该液体位于检查窗上(见图 2)。
•
如果液体的颜色不对、混浊或含有大的颗粒,不要使用该预充式注射器。如果发生
这种情况,请向您的医生或药剂师说明。

2.
选择和准备注射部位(见图 3)
•
•
•
通常将该药注入大腿中部前侧。
还可以在肚脐下的下腹部(腹部),除肚脐正下方 5 cm的面积外注射。
不要在皮肤触痛、挫伤、红肿、呈鳞片状、发硬的区域内或有疤痕或妊娠纹的区
域内注射。
•
如果一次给药需要进行多次注射,则在身体不同部位进行。
图3
护理人员注射部位的选择(见图 4)
•
•
如果有护理人员给您注射,他们还可以在上臂的外侧区域注射。
同样的,无论您的体形或身高如何,上述的所有部位均可用于注射。

图4
准备注射部位
•
•
•
用肥皂和温水彻底地洗手。
用酒精棉签擦拭该注射部位。
注射前使皮肤干燥。不要在洁净区域上扇风或吹风。
在进行注射之前,切勿再次碰触此部位。
3.
注射本品
准备好注射本品之前不能取下针头保护帽。取下针头保护帽后,应在 5分钟内注射
该药物。
取针头保护帽的过程中,请勿触摸柱塞。
取下针头保护帽(见图 5)
•
•
•
当您准备好注射时,用一只手握住注射器的主体。
将针头保护帽直拉取下后扔掉。进行此操作时切勿碰触柱塞。
您可能会注意到在预充式注射器中有气泡或在针头的底部有一滴液体。两种情况
均属正常,无需去除。
•
取下针头保护帽后立即注射药物。
不要碰触针头或使其接触任何表面。
如果预充式注射器跌落,而针头保护帽未盖在针头上,请勿使用。如果发生此情况,
请联系您的医生或药剂师。
图 5
定位预充式注射器进行注射
•
用一只手的中指和食指握住预充式注射器的主体,将拇指放在柱塞头的顶部,并
用另一只手轻轻捏住您之前清洁过的皮肤区域。握稳。
在任何时候都切勿向后拉柱塞。
注射本品
• 针头与捏住的皮肤呈约 45度角。一次性迅速将针头尽可能深地插入皮肤(见图 6)。
图 6

•
推动柱塞将所有药物注入体内,直到柱塞头完全推进两个针头防护翼之间(见图
7)。
图 7
•
当柱塞推到底时,保持施加在柱塞头上的压力,将针头从皮肤上取出(见图 8)。
图 8
•
慢慢将拇指移离柱塞头,以允许空注射器向上移动,直至针头防护装置覆盖整个
针头,如图 9所示:
图 9

4.
注射之后
使用棉球或纱布
•
•
•
注射部位可能有少量出血或渗出液体。这是正常现象。
您可以将绵球或纱布按在注射部位上,持续 10秒。
如有必要,您可以在注射部位上贴上一小块创可贴。
不能摩擦皮肤。
丢弃本品注射器(见图 10)
•
立即将您使用过的预充式注射器放入一个锐器盒内。确保按照医生或护士的指示
处置锐器盒。
不要试图再次盖上该针头。
为了您和他人的安全和健康,请勿再次使用同一支预充式注射器。
如果觉得注射出现了错误或者您不确定,请向您的医生或药剂师说明。
图 10

Simponi
Generic Name: golimumab
Dosage Form: injection, solution
Medically reviewed by Drugs.com. Last updated on Sep 1, 2019.
WARNING: SERIOUS INFECTIONS AND MALIGNANCY
SERIOUS INFECTIONS
Patients treated with Simponi® are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue Simponi if a patient develops a serious infection.
Reported infections with TNF blockers, of which Simponi is a member, include:
Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Test patients for latent tuberculosis before Simponi use and during therapy. Initiate treatment for latent TB prior to Simponi use.Invasive fungal infections including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric antifungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria.Consider the risks and benefits of treatment with Simponi prior to initiating therapy in patients with chronic or recurrent infection.
Monitor patients closely for the development of signs and symptoms of infection during and after treatment with Simponi, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see Warnings and Precautions (5.1)].
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, of which Simponi is a member [see Warnings and Precautions (5.2)].
Simponi, in combination with methotrexate, is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis.
Psoriatic ArthritisSimponi, alone or in combination with methotrexate, is indicated for the treatment of adult patients with active psoriatic arthritis.
Ankylosing SpondylitisSimponi is indicated for the treatment of adult patients with active ankylosing spondylitis.
Ulcerative ColitisSimponi is indicated in adult patients with moderately to severely active ulcerative colitis who have demonstrated corticosteroid dependence or who have had an inadequate response to or failed to tolerate oral aminosalicylates, oral corticosteroids, azathioprine, or 6-mercaptopurine for:
inducing and maintaining clinical responseimproving endoscopic appearance of the mucosa during inductioninducing clinical remissionachieving and sustaining clinical remission in induction responders [see Clinical Studies (14.4)].Simponi Dosage and AdministrationDosage in Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing SpondylitisThe Simponi dose regimen is 50 mg administered by subcutaneous injection once a month.
For patients with rheumatoid arthritis (RA), Simponi should be given in combination with methotrexate and for patients with psoriatic arthritis (PsA) or ankylosing spondylitis (AS), Simponi may be given with or without methotrexate or other nonbiologic Disease-Modifying Antirheumatic Drugs (DMARDs). For patients with RA, PsA, or AS, corticosteroids, non-biologic DMARDs, and/or NSAIDs may be continued during treatment with Simponi.
Dosage in Moderately to Severely Active Ulcerative ColitisThe recommended Simponi induction dosage regimen is a 200-mg subcutaneous injection at Week 0, followed by 100 mg at Week 2, and then maintenance therapy with 100 mg every 4 weeks.
Monitoring to Assess SafetyPrior to initiating Simponi and periodically during therapy, evaluate patients for active tuberculosis and tested for latent infection [see Warnings and Precautions (5.1)]. Prior to initiating Simponi, patients should be tested for hepatitis B viral infection [see Warnings and Precautions (5.1)].
Simponi is intended for use under the guidance and supervision of a healthcare provider. After proper training in subcutaneous injection technique, a patient may self-inject with Simponi if a physician determines that it is appropriate. Instruct patients to follow the directions provided below [see Instructions for Use]:
To ensure proper use, allow the prefilled syringe or autoinjector to sit at room temperature outside the carton for at least 30 minutes prior to subcutaneous injection. Do not warm Simponi in any other way.Prior to administration, visually inspect the solution for particles and discoloration through the viewing window. Simponi is clear to slightly opalescent and colorless to light yellow. Do not use Simponi, if the solution is discolored, or cloudy, or if foreign particles are present.Do not use any leftover product remaining in the prefilled syringe or prefilled autoinjector.Instruct patients sensitive to latex not to handle the needle cover on the prefilled syringe or the needle cover of the prefilled syringe within the autoinjector cap because it contains dry natural rubber (a derivative of latex).At the time of dosing, if multiple injections are required, administer the injections at different sites on the body.Rotate injection sites and never give injections into areas where the skin is tender, bruised, red, or hard.Dosage Forms and StrengthsInjection: 50 mg/0.5 mL and 100 mg/mL clear to slightly opalescent, colorless to light yellow solution in a single-dose prefilled syringe or single-dose SmartJect autoinjector.
ContraindicationsNone.
Warnings and PrecautionsSerious InfectionsPatients treated with Simponi are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, or parasitic organisms including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis, and tuberculosis have been reported with TNF blockers. Patients have frequently presented with disseminated rather than localized disease. The concomitant use of a TNF blocker and abatacept or anakinra was associated with a higher risk of serious infections; therefore, the concomitant use of Simponi and these biologic products is not recommended [see Warnings and Precautions (5.6, 5.7) and Drug Interactions (7.2)].
Treatment with Simponi should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions and/or patients taking concomitant immunosuppressants such as corticosteroids or methotrexate may be at greater risk of infection. Consider the risks and benefits of treatment prior to initiating Simponi in patients:
with chronic or recurrent infection;who have been exposed to tuberculosis;with a history of an opportunistic infection;who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; orwith underlying conditions that may predispose them to infection.Monitoring
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with Simponi. Discontinue Simponi if a patient develops a serious infection, an opportunistic infection, or sepsis. For a patient who develops a new infection during treatment with Simponi, perform a prompt and complete diagnostic workup appropriate for an immunocompromised patient, initiate appropriate antimicrobial therapy, and closely monitor them.
Serious Infection in Clinical Trials
In controlled Phase 3 trials through Week 16 in patients with RA, PsA, and AS, serious infections were observed in 1.4% of Simponi-treated patients and 1.3% of control-treated patients. In the controlled Phase 3 trials through Week 16 in patients with RA, PsA, and AS, the incidence of serious infections per 100 patient-years of follow-up was 5.7 (95% CI: 3.8, 8.2) for the Simponi group and 4.2 (95% CI: 1.8, 8.2) for the placebo group. In the controlled Phase 2/3 trial through Week 6 of Simponi induction in UC, the incidence of serious infections in Simponi 200/100 mg-treated patients was similar to the incidence of serious infections in placebo-treated patients. Through Week 60, the incidence of serious infections was similar in patients who received Simponi induction and 100 mg during maintenance compared with patients who received Simponi induction and placebo during the maintenance portion of the UC trial. Serious infections observed in Simponi-treated patients included sepsis, pneumonia, cellulitis, abscess, tuberculosis, invasive fungal infections, and hepatitis B infection.
Tuberculosis
Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving TNF blockers, including patients who have previously received treatment for latent or active tuberculosis. Evaluate patients for tuberculosis risk factors and test for latent infection prior to initiating Simponi and periodically during therapy.
Treatment of latent tuberculosis infection prior to therapy with TNF blockers has been shown to reduce the risk of tuberculosis reactivation during therapy. Prior to initiating Simponi, assess if treatment for latent tuberculosis is needed; an induration of 5 mm or greater is a positive tuberculin skin test, even for patients previously vaccinated with Bacille Calmette-Guerin (BCG).
Consider anti-tuberculosis therapy prior to initiation of Simponi in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Cases of active tuberculosis have occurred in patients treated with Simponi during and after treatment for latent tuberculosis. Monitor patients for the development of signs and symptoms of tuberculosis including patients who tested negative for latent tuberculosis infection prior to initiating therapy, patients who are on treatment for latent tuberculosis, or patients who were previously treated for tuberculosis infection.
Consider tuberculosis in the differential diagnosis in patients who develop a new infection during Simponi treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
In the controlled and uncontrolled portions of the Phase 2 RA and Phase 3 RA, PsA, and AS trials, the incidence of active TB was 0.23 and 0 per 100 patient-years in 2347 Simponi-treated patients and 674 placebo-treated patients, respectively. Cases of TB included pulmonary and extrapulmonary TB. The overwhelming majority of the TB cases occurred in countries with a high incidence rate of TB. In the controlled Phase 2/3 trial of Simponi induction through Week 6 in UC, no cases of TB were observed in Simponi 200/100 mg-treated patients or in placebo-treated patients. Through Week 60, the incidence per 100 patient-years of TB in patients who received Simponi induction and 100 mg during the maintenance portion of the UC trial was 0.52 (95% CI: 0.11, 1.53). One case of TB was observed in the placebo maintenance group in a patient who received Simponi intravenous (IV) induction.
Invasive Fungal Infections
If patients develop a serious systemic illness and they reside or travel in regions where mycoses are endemic, consider invasive fungal infection in the differential diagnosis. Consider appropriate empiric antifungal therapy, and take into account both the risk for severe fungal infection and the risks of antifungal therapy while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. To aid in the management of such patients, consider consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections.
Hepatitis B Virus Reactivation
The use of TNF blockers including Simponi has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic hepatitis B carriers (i.e., surface antigen positive). In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of these reports have occurred in patients who received concomitant immunosuppressants.
All patients should be tested for HBV infection before initiating TNF-blocker therapy. For patients who test positive for hepatitis B surface antigen, consultation with a physician with expertise in the treatment of hepatitis B is recommended before initiating TNF-blocker therapy. The risks and benefits of treatment should be considered prior to prescribing TNF blockers, including Simponi, to patients who are carriers of HBV. Adequate data are not available on whether antiviral therapy can reduce the risk of HBV reactivation in HBV carriers who are treated with TNF blockers. Patients who are carriers of HBV and require treatment with TNF blockers should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy.
In patients who develop HBV reactivation, TNF blockers should be stopped and antiviral therapy with appropriate supportive treatment should be initiated. The safety of resuming TNF blockers after HBV reactivation has been controlled is not known. Therefore, prescribers should exercise caution when considering resumption of TNF blockers in this situation and monitor patients closely.
MalignanciesMalignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy ≤ 18 years of age), of which Simponi is a member. Approximately half the cases were lymphomas, including Hodgkin's and non-Hodgkin's lymphoma. The other cases represented a variety of malignancies, including rare malignancies that are usually associated with immunosuppression, and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months (range 1 to 84 months) after the first dose of TNF-blocker therapy. Most of the patients were receiving concomitant immunosuppressants. These cases were reported postmarketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports.
The risks and benefits of TNF-blocker treatment, including Simponi, should be considered prior to initiating therapy in patients with a known malignancy other than a successfully treated nonmelanoma skin cancer (NMSC) or when considering continuing a TNF-blocker in patients who develop a malignancy.
In the controlled portions of clinical trials of TNF blockers, including Simponi, more cases of lymphoma have been observed among patients receiving anti-TNF treatment compared with patients in the control groups. During the controlled portions of the Phase 2 trials in RA, and the Phase 3 trials in RA, PsA and AS, the incidence of lymphoma per 100 patient-years of follow-up was 0.21 (95% CI: 0.03, 0.77) in the combined Simponi group compared with an incidence of 0 (95% CI: 0, 0.96) in the placebo group. In the controlled and uncontrolled portions of these clinical trials in 2347 Simponi-treated patients with a median follow-up of 1.4 years, the incidence of lymphoma was 3.8-fold higher than expected in the general U.S. population according to the SEER database (adjusted for age, gender, and race).1 Through Week 60 of the UC trials, there were no cases of lymphoma with Simponi. Patients with RA and other chronic inflammatory diseases, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at higher risk (up to several fold) than the general population for the development of lymphoma, even in the absence of TNF-blocking therapy. Cases of acute and chronic leukemia have been reported with TNF-blocker use, including Simponi, in rheumatoid arthritis and other indications. Even in the absence of TNF-blocker therapy, patients with rheumatoid arthritis may be at a higher risk (approximately 2-fold) than the general population for the development of leukemia.
Rare postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL) have been reported in patients treated with TNF-blocking agents. This rare type of T-cell lymphoma has a very aggressive disease course and is usually fatal. Nearly all of the reported TNF blocker associated cases have occurred in patients with Crohn's disease or ulcerative colitis. The majority were in adolescent and young adult males. Almost all these patients had received treatment with azathioprine (AZA) or 6-mercaptopurine (6–MP) concomitantly with a TNF blocker at or prior to diagnosis. The potential risk with the combination of AZA or 6-MP and Simponi should be carefully considered. A risk for the development for hepatosplenic T-cell lymphoma in patients treated with TNF blockers cannot be excluded.
During the controlled portions of the Phase 2 trial in RA, and the Phase 3 trials in RA, PsA and AS, the incidence of malignancies other than lymphoma per 100 patient-years of follow-up was not elevated in the combined Simponi group compared with the placebo group. In the controlled and uncontrolled portions of these trials, the incidence of malignancies, other than lymphoma, in Simponi-treated patients was similar to that expected in the general U.S. population according to the SEER database (adjusted for age, gender, and race).1 In the 6-week placebo-controlled portions of the Simponi Phase 2/3 clinical trials in UC, the incidence of non-lymphoma malignancies (excluding nonmelanoma skin cancer) was similar between the Simponi and the placebo group. Through Week 60, the incidence of non-lymphoma malignancies (excluding nonmelanoma skin cancer) was similar to the general U.S. population according to the SEER database (adjusted for age, gender, and race).1 Short follow-up periods, such as those of one year or less in the studies above, may not adequately reflect the true incidence of malignancies.
It is not known if Simponi treatment influences the risk for developing dysplasia or colon cancer. All patients with ulcerative colitis who are at increased risk for dysplasia or colon carcinoma (for example, patients with long-standing ulcerative colitis or primary sclerosing cholangitis), or who had a prior history of dysplasia or colon carcinoma should be screened for dysplasia at regular intervals before therapy and throughout their disease course. This evaluation should include colonoscopy and biopsies per local recommendations. In patients with newly diagnosed dysplasia treated with Simponi, the risks and benefits to the individual patient must be carefully reviewed and consideration should be given to whether therapy should be continued.
Melanoma and Merkel cell carcinoma have been reported in patients treated with TNF-blocking agents, including Simponi. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer.
In controlled trials of other TNF blockers in patients at higher risk for malignancies (e.g., patients with chronic obstructive pulmonary disease [COPD], patients with Wegener's granulomatosis treated with concomitant cyclophosphamide) a greater portion of malignancies occurred in the TNF-blocker group compared to the controlled group. In an exploratory 1-year clinical trial evaluating the use of 50 mg, 100 mg, and 200 mg of Simponi in 309 patients with severe persistent asthma, 6 patients developed malignancies other than NMSC in the Simponi groups compared to none in the control group. Three of the 6 patients were in the 200-mg Simponi group.
Congestive Heart FailureCases of worsening congestive heart failure (CHF) and new onset CHF have been reported with TNF blockers, including Simponi. Some cases had a fatal outcome. In several exploratory trials of other TNF blockers in the treatment of CHF, there were greater proportions of TNF-blocker-treated patients who had CHF exacerbations requiring hospitalization or increased mortality. Simponi has not been studied in patients with a history of CHF and Simponi should be used with caution in patients with CHF. If a decision is made to administer Simponi to patients with CHF, these patients should be closely monitored during therapy, and Simponi should be discontinued if new or worsening symptoms of CHF appear.
Use of TNF blockers, of which Simponi is a member, has been associated with rare cases of new onset or exacerbation of central nervous system (CNS) demyelinating disorders, including multiple sclerosis (MS) and peripheral demyelinating disorders, including Guillain-Barré syndrome. Cases of central demyelination, MS, optic neuritis, and peripheral demyelinating polyneuropathy have rarely been reported in patients treated with Simponi [see Adverse Reactions (6.1)]. Prescribers should exercise caution in considering the use of TNF blockers, including Simponi, in patients with central or peripheral nervous system demyelinating disorders. Discontinuation of Simponi should be considered if these disorders develop.
AutoimmunityTreatment with TNF blockers, including Simponi, may result in the formation of antinuclear antibodies (ANA) and, rarely, in the development of a lupus-like syndrome [see Adverse Reactions (6.1)]. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with Simponi, treatment should be discontinued.
Use with AbataceptIn controlled trials, the concurrent administration of another TNF blocker and abatacept was associated with a greater proportion of serious infections than the use of a TNF blocker alone; and the combination therapy, compared to the use of a TNF blocker alone, has not demonstrated improved clinical benefit in the treatment of RA. Therefore, the combination of TNF blockers, including Simponi, and abatacept is not recommended [see Drug Interactions (7.2)].
Use with AnakinraConcurrent administration of anakinra (an interleukin-1 antagonist) and another TNF blocker was associated with a greater portion of serious infections and neutropenia and no additional benefits compared with the TNF-blocker alone. Therefore, the combination of anakinra with TNF blockers, including Simponi, is not recommended [see Drug Interactions (7.2)].
Switching Between Biological Disease-Modifying Antirheumatic DrugsCare should be taken when switching from one biological product to another biological product since overlapping biological activity may further increase the risk of infection.
Hematologic CytopeniasThere have been reports of pancytopenia, leukopenia, neutropenia, agranulocytosis, aplastic anemia, and thrombocytopenia in patients receiving golimumab. Caution should be exercised when using TNF blockers, including Simponi, in patients who have or have had significant cytopenias.
Vaccinations/Therapeutic Infectious AgentsLive Vaccines
Patients treated with Simponi may receive vaccinations, except for live vaccines. In patients receiving anti-TNF therapy, limited data are available on the response to live vaccination, or on the secondary transmission of infection by live vaccines. Use of live vaccines could result in clinical infections, including disseminated infections.
Therapeutic Infectious Agents
Other uses of therapeutic infectious agents such as live attenuated bacteria (e.g., BCG bladder instillation for the treatment of cancer) could result in clinical infections, including disseminated infections. It is recommended that therapeutic infectious agents not be given concurrently with Simponi.
Non-live Vaccines
In the Phase 3 PsA trial, after pneumococcal vaccination, a similar proportion of Simponi-treated and placebo-treated patients were able to mount an adequate immune response of at least a 2-fold increase in antibody titers to pneumococcal polysaccharide vaccine. In both Simponi-treated and placebo-treated patients, the proportions of patients with response to pneumococcal vaccine were lower among patients receiving MTX compared with patients not receiving MTX. The data suggest that Simponi does not suppress the humoral immune response to the pneumococcal vaccine.
Hypersensitivity ReactionsIn postmarketing experience, serious systemic hypersensitivity reactions (including anaphylactic reaction) have been reported following Simponi administration. Some of these reactions occurred after the first administration of Simponi. If an anaphylactic or other serious allergic reaction occurs, administration of Simponi should be discontinued immediately and appropriate therapy instituted.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety data described below are based on 5 pooled, randomized, double-blind, controlled Phase 3 trials in patients with RA, PsA, and AS (Trials RA-1, RA-2, RA-3, PsA, and AS) [see Clinical Studies (14.1, 14.2, and 14.3)]. These 5 trials included 639 control-treated patients and 1659 Simponi-treated patients including 1089 with RA, 292 with PsA, and 278 with AS. The safety data in 1233 Simponi-treated patients with ulcerative colitis from 3 pooled, randomized, double-blind, controlled Phase 2/3 trials are also described below (Trials UC-1, UC-2, and UC-3) [see Clinical Studies (14.4)]. The proportion of patients who discontinued treatment due to adverse reactions in the controlled Phase 3 trials through Week 16 in RA, PsA and AS was 2% for Simponi-treated patients and 3% for placebo-treated patients. The most common adverse reactions leading to discontinuation of Simponi in the controlled Phase 3 trials in RA, PsA and AS through Week 16 were sepsis (0.2%), alanine aminotransferase increased (0.2%), and aspartate aminotransferase increased (0.2%). The most common adverse drug reactions leading to discontinuation through Week 60 of the UC trials in patients who received Simponi induction and 100 mg during maintenance compared with patients who received Simponi induction and placebo during maintenance were tuberculosis (0.3% vs. 0.6%) and anemia (0.3% vs. 0%), respectively.
The most serious adverse reactions were:
Serious Infections [see Warnings and Precautions (5.1)]Malignancies [see Warnings and Precautions (5.2)]Upper respiratory tract infection and nasopharyngitis were the most common adverse reactions reported in the combined Phase 3 RA, PsA and AS trials through Week 16, occurring in 7% and 6% of Simponi-treated patients as compared with 6% and 5% of control-treated patients, respectively.
Infections
In controlled Phase 3 trials through Week 16 in RA, PsA, and AS, infections were observed in 28% of Simponi-treated patients compared to 25% of control-treated patients. For serious infections, see the Warnings and Precautions section [see Warnings and Precautions (5.1)]. In the controlled Phase 2/3 trial of Simponi induction through Week 6 in UC, the rates of infections were similar in Simponi 200/100 mg-treated patients and placebo-treated patients, or approximately 12%. Through Week 60, the incidence per patient year of infections was similar in patients who received Simponi induction and 100 mg during maintenance compared with patients who received Simponi induction and placebo during the maintenance portion of the UC trial.
Demyelinating Disorders
In the controlled Phase 2/3 trial of Simponi induction through Week 6, no cases of demyelination were observed in Simponi 200/100 mg-treated patients or placebo-treated patients. Through Week 60, there were no cases of demyelination in the Simponi 100-mg group during maintenance. One case of CNS demyelination was observed in the placebo maintenance group in a patient who received Simponi 400/200 mg during induction.
Liver Enzyme Elevations
There have been reports of severe hepatic reactions including acute liver failure in patients receiving TNF blockers. In controlled Phase 3 trials of Simponi in patients with RA, PsA, and AS through Week 16, ALT elevations ≥ 5 × ULN occurred in 0.2% of control-treated patients and 0.7% of Simponi-treated patients and ALT elevations ≥ 3 × ULN occurred in 2% of control-treated patients and 2% of Simponi-treated patients. Since many of the patients in the Phase 3 trials for RA, PsA, and AS were also taking medications that cause liver enzyme elevations (e.g., NSAIDs, MTX), the relationship between Simponi and liver enzyme elevation is not clear.
In Phase 2/3 UC trials, the incidence of ALT elevations ≥ 5 × ULN was similar in Simponi-treated patients and placebo-treated patients, or approximately 1%, with an average duration of follow-up of 46 weeks and 18 weeks, respectively. ALT elevations ≥ 3 × ULN occurred in 2.0% of Simponi-treated patients compared with 1.5% of placebo-treated patients with an average duration of follow-up of 46 weeks and 18 weeks, respectively.
Autoimmune Disorders and Autoantibodies
In the controlled Phase 3 trials in patients with RA, PsA, and AS through Week 14, there was no association of Simponi treatment and the development of newly positive anti-dsDNA antibodies. In Phase 3 trials in RA, PsA, and AS through 1 year of follow-up, 4.0% of Simponi-treated patients and 2.6% of control patients were newly antinuclear antibody (ANA)-positive (at titers of 1:160 or greater). The frequency of anti-dsDNA antibodies at 1 year of follow-up was uncommon in patients who were anti-dsDNA negative at baseline. Through Week 60 of the UC trials, 3.5% of patients who received Simponi induction and 100 mg during maintenance were newly ANA-positive (at titers of 1:160 or greater) compared with 3.5% of patients who received Simponi induction and placebo during the maintenance portion of the UC trial. The frequency of anti-dsDNA antibodies at 1 year of follow-up in patients who were anti-dsDNA negative at baseline was 0.5% in patients receiving Simponi induction and 100 mg during maintenance compared with 0% in patients who received Simponi induction and placebo during maintenance [see Warnings and Precautions (5.5)].
Injection Site Reactions
In controlled Phase 3 trials through Week 16 in RA, PsA and AS, 6% of Simponi-treated patients had injection site reactions compared with 2% of control-treated patients. The majority of the injection site reactions were mild and the most frequent manifestation was injection site erythema.
In the controlled Phase 2/3 trial through Week 6 in UC, 3.4% of Simponi-treated patients had injection site reactions compared with 1.5% in control-treated patients. The majority of the injection site reactions were mild and moderate and the most frequent manifestation was injection site erythema.
In controlled Phase 2 and 3 trials in RA, PsA, AS, and Phase 2/3 UC trials, no patients treated with Simponi developed anaphylactic reactions.
Other Adverse Reactions
Table 1 summarizes the adverse drug reactions that occurred at a rate of at least 1% in the Simponi ± DMARD group and with a higher incidence than in the placebo ± DMARD group during the controlled period of the 5 pooled Phase 3 trials through Week 16 in patients with RA, PsA, and AS.
| Simponi ± DMARDs | Placebo ± DMARDs | |
|---|---|---|
| *Patients may have taken concomitant MTX, sulfasalazine, hydroxychloroquine, low dose corticosteroids (≤ 10 mg of prednisone/day or equivalent), and/or NSAIDs during the trials). | ||
| Patients treated | 1659 | 639 |
| Adverse Reaction | ||
| Infections and infestations | ||
| Upper respiratory tract infection (nasopharyngitis, pharyngitis, laryngitis, and rhinitis) | 16% | 13% |
| Viral infections (such as influenza and herpes) | 5% | 3% |
| Bronchitis | 2% | 1% |
| Superficial fungal infections | 2% | 1% |
| Sinusitis | 2% | 1% |
| General disorders and administration site conditions | ||
| Injection site reaction (injection site erythema, urticaria, induration, pain, bruising, pruritus, irritation, paresthesia) | 6% | 2% |
| Investigations | ||
| Alanine aminotransferase increased | 4% | 3% |
| Aspartate aminotransferase increased | 3% | 2% |
| Vascular disorders | ||
| Hypertension | 3% | 2% |
| Nervous system disorders | ||
| Dizziness | 2% | 1% |
| Paresthesia | 2% | 1% |
| Gastrointestinal disorders | ||
| Constipation | 1% | <1% |
Less Common Clinical Trial Adverse Drug Reactions
Adverse drug reactions that occurred <1% in Simponi-treated patients during the Simponi clinical trials that do not appear in the Warnings and Precautions section included the following events listed by system organ class:
Infections and infestations: Septic shock, atypical mycobacterial infection, pyelonephritis, arthritis bacterial, bursitis infective
Neoplasms benign, malignant and unspecified: Leukemia
Skin and subcutaneous tissue disorders: Psoriasis (new onset or worsening, palmar/plantar and pustular), vasculitis (cutaneous)
Vascular disorders: Vasculitis (systemic)
Other Clinical Trial Adverse Drug Reactions in Ulcerative Colitis Clinical Trials
In the Phase 2/3 trials in UC evaluating 1233 Simponi-treated patients, no new adverse drug reactions were identified and the frequency of adverse drug reactions was similar to the safety profile observed in patients with RA, PsA and AS.
ImmunogenicityAs with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to golimumab in the trials described below with the incidence of antibodies in other trials or to other products may be misleading.
Results from the EIA Method
Using an enzyme immunoassay (EIA method), antibodies to golimumab were detected in 57 (4%) of Simponi-treated patients across the Phase 3 RA, PsA and AS trials through Week 24. Similar rates were observed in each of the 3 indications. Patients who received Simponi with concomitant MTX had a lower proportion of antibodies to golimumab than patients who received Simponi without MTX (approximately 2% vs. 7%, respectively).
With the EIA method, the presence of serum concentrations of golimumab can interfere with the detection of antibodies to golimumab leading to inconclusive results. In UC trials, 34 (3%), 341 (28%) and 823 (69%) of Simponi-treated patients were positive, negative and inconclusive for antibodies to golimumab, respectively. Treatment with concomitant immunomodulators (AZA, 6-MP or MTX) resulted in a lower proportion of patients with antibodies to golimumab than patients receiving Simponi without immunomodulators (2% vs. 4%, respectively).
Of the patients with a positive antibody response to golimumab in the Phase 2 and 3 trials, most were determined to have neutralizing antibodies to golimumab as measured by a cell-based functional assay.
Results from the Drug-Tolerant EIA Method
A drug-tolerant enzyme immunoassay (drug-tolerant EIA) method for detecting antibodies to golimumab was developed and validated, which eliminated the inconclusive category as reported above. This method is approximately 16-fold more sensitive than the original EIA method with less interference from golimumab in serum.
Based on the drug tolerant EIA method, 246 (23%) of Simponi-treated patients across the Phase 3 RA, PsA and AS trials, antibodies to golimumab were detected in 59 (16%), 106 (28%), and 81 (24%) patients, respectively. Treatment with concomitant MTX resulted in a lower proportion of patients with antibodies to golimumab than in patients receiving Simponi without MTX in RA patients (7% vs. 35%), in PsA patients (18% vs. 38%) and in AS patients (6% vs. 29%). A trend of decreasing drug concentrations with increasing antibody titers was observed. While an overall decrease in clinical efficacy for ADA positive patients compared with ADA negative patients was not observed in patients with RA (ACR 20: 75% vs. 75%), PsA (ACR 20: 72% vs. 66%) and AS (ASAS 20: 57% vs. 65%), higher titer antibodies may be associated with diminished efficacy.
In the UC trials, 254 (21%) of Simponi-treated patients were positive for antibodies to golimumab through week 54 while the remaining 941 (79%) patients were negative. Treatment with concomitant immunomodulators (AZA, 6-MP or MTX) in the UC trials resulted in a lower proportion of patients with antibodies to golimumab than in patients receiving Simponi without immunomodulators (12% vs. 26%). There is a trend of decreasing drug concentrations with increasing antibody titers. Although the development of antibodies to golimumab did not preclude clinical response, a trend toward decreased efficacy in ADA positive patients was observed compared to ADA negative patients in the UC trials (clinical response 38% vs. 53%).
Postmarketing ExperienceThe following adverse reactions have been identified during post-approval use of golimumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Simponi exposure.
Immune system disorders: Serious systemic hypersensitivity reactions (including anaphylactic reaction) [see Warnings and Precautions (5.11)], sarcoidosis
Neoplasms benign, malignant and unspecified: Melanoma, Merkel cell carcinoma [see Warnings and Precautions (5.2)]
Respiratory, thoracic and mediastinal disorders: Interstitial lung disease
Skin and subcutaneous tissue disorders: Skin exfoliation, lichenoid reactions, rash, bullous skin reactions
Drug InteractionsMethotrexateFor the treatment of RA, Simponi should be used with methotrexate (MTX) [see Clinical Studies (14.1)]. Since the presence or absence of concomitant MTX did not appear to influence the efficacy or safety of Simponi in the treatment of PsA or AS, Simponi can be used with or without MTX in the treatment of PsA and AS [see Clinical Studies (14.2, 14.3) and Clinical Pharmacology (12.3)].
Biological Products for RA, PsA, and/or ASAn increased risk of serious infections has been seen in clinical RA trials of other TNF blockers used in combination with anakinra or abatacept, with no added benefit; therefore, use of Simponi with abatacept or anakinra is not recommended [see Warnings and Precautions (5.6, 5.7)]. A higher rate of serious infections has also been observed in RA patients treated with rituximab who received subsequent treatment with a TNF blocker. The concomitant use of Simponi with biologics approved to treat RA, PsA, or AS is not recommended because of the possibility of an increased risk of infection.
Live Vaccines/Therapeutic Infectious AgentsLive vaccines should not be given concurrently with Simponi [see Warnings and Precautions (5.10)].
Therapeutic infectious agents should not be given concurrently with Simponi [see Warnings and Precautions (5.10)].
Infants born to women treated with Simponi during their pregnancy may be at increased risk of infection for up to 6 months. Administration of live vaccines to infants exposed to Simponi in utero is not recommended for 6 months following the mother's last Simponi injection during pregnancy [see Use in Specific Populations (8.1)].
Cytochrome P450 SubstratesThe formation of CYP450 enzymes may be suppressed by increased levels of cytokines (e.g., TNFα) during chronic inflammation. Therefore, it is expected that for a molecule that antagonizes cytokine activity, such as golimumab, the formation of CYP450 enzymes could be normalized. Upon initiation or discontinuation of Simponi in patients being treated with CYP450 substrates with a narrow therapeutic index, monitoring of the effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) is recommended and the individual dose of the drug product may be adjusted as needed.
USE IN SPECIFIC POPULATIONSPregnancyRisk Summary
There are no adequate and well-controlled trials of Simponi in pregnant women. Monoclonal antibodies, such as golimumab, are transported across the placenta during the third trimester of pregnancy and may affect immune response in the in utero exposed infant [see Clinical Considerations]. In an animal reproductive study, golimumab administered by the subcutaneous route to pregnant monkeys, during the period of organogenesis, at doses that produced exposures approximately 360 times the maximum recommended human dose (MRHD) had no adverse fetal effects [see Data]. In a pre- and post-natal development study with pregnant monkeys, subcutaneous administration of golimumab, during the later gestational and lactation periods, at doses producing maximal maternal blood concentrations approximately 460 times those found with the MRHD had no adverse developmental effects on infants [see Data]. Simponi should be used during pregnancy only if clearly needed.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and of miscarriage is 15–20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Golimumab crosses the placenta during pregnancy. Another TNF-blocking monoclonal antibody administered during pregnancy was detected for up to 6 months in the serum of infants. Consequently, these infants may be at increased risk of infection. Administration of live vaccines to infants exposed to Simponi in utero is not recommended for 6 months following the mother's last Simponi injection during pregnancy [see Warnings and Precautions (5.10) and Drug Interactions (7.3)].
Data
Human Data
Limited data on use of Simponi in pregnant women from observational studies, published case reports, and postmarketing surveillance are insufficient to inform a drug associated risk.
Animal Data
In an embryofetal developmental toxicology study in which pregnant cynomolgus monkeys were treated with golimumab during the period organogenesis from gestation days (GD) 20 to 51, exposures up to 360 times greater than the exposure at the MRHD (on an area under the curve (AUC) basis with maternal subcutaneous doses up to 50 mg/kg twice weekly) produced no evidence of fetal malformations or embryotoxicity. There was no evidence of maternal toxicity. Umbilical cord blood samples collected at the end of the second trimester showed that fetuses were exposed to golimumab during gestation.
In a pre- and postnatal developmental study in which pregnant cynomolgus monkeys were treated with golimumab from gestation day 50 to postpartum day 33, maximal drug concentrations approximately 460 times greater than that found with the MRHD (on a maximum blood concentration (Cmax) basis at steady-state with maternal subcutaneous doses up to 50 mg/kg twice weekly) were not associated with any evidence of developmental defects in infants. There was no evidence of maternal toxicity. Golimumab was present in fetal serum at the end of the second trimester and in neonatal serum from the time of birth and for up to 6 months postpartum.
LactationRisk Summary
There is no information regarding the presence of Simponi in human milk, the effects on breastfed infants, or the effects on milk production. Maternal IgG is known to be present in human milk. If golimumab is transferred into human milk, the effects of local exposure in the gastrointestinal tract and potential limited systemic exposure in the infant to golimumab are unknown. The developmental and health benefits of breast-feeding should be considered along with the mother's clinical need for Simponi and any potential adverse effects on the breast-fed infants from Simponi, or from the underlying maternal condition.
Data
Animal Data
In the pre- and postnatal development study in cynomolgus monkeys in which golimumab was administered subcutaneously during pregnancy and lactation, golimumab was detected in the breast milk at concentrations that were approximately 400-fold lower than the maternal serum concentrations.
Pediatric UseEffectiveness of Simponi in pediatric patients less than 18 years of age has not been established.
The safety and efficacy of Simponi were evaluated in a multicenter, placebo-controlled, double-blind, randomized-withdrawal, parallel group study in 173 children (2 to 17 years of age) with active polyarticular juvenile idiopathic arthritis (pJIA) despite treatment with MTX for at least 3 months. Subjects were maintained on their stable dose of MTX at the same dose (mg/week) at study entry. Concurrent use of stable doses of oral corticosteroids (≤10 mg/day or 0.2 mg/kg/day prednisone or equivalent, whichever was less) and/or NSAIDs was permitted. In the 16 week open-label phase, all patients received MTX and Simponi 30 mg/m2 (maximum 50 mg) subcutaneously every 4 weeks. Patients who achieved an ACR Ped 30 response at Week 16 entered the randomized-withdrawal phase of the study and received MTX and either Simponi 30 mg/m2 (maximum 50 mg) or placebo every 4 weeks through Week 48.
The primary endpoint of the study was the proportion of patients who did not experience a flare between Week 16 and Week 48, among all subjects who entered the randomized withdrawal phase. The efficacy of Simponi in the treatment of pJIA was not demonstrated in this study because there was no statistical evidence of differences in flare rate between Simponi-treated patients and placebo patients between Weeks 16 and 48.
In this study, the frequency and type of the adverse reactions seen in children were generally similar to those observed in adults.
Geriatric UseIn the Phase 3 trials in RA, PsA, and AS, there were no overall differences in SAEs, serious infections, and AEs in Simponi-treated patients ages 65 or older (N=155) compared with younger Simponi-treated patients. In UC, there were insufficient numbers of patients aged 65 and over to determine whether they respond differently from patients aged 18 to 65. Because there is a higher incidence of infections in the geriatric population in general, caution should be used in treating geriatric patients with Simponi.
In a clinical trial, 5 patients received protocol-directed single infusions of 10 mg/kg of intravenous Simponi without serious adverse reactions or other significant reactions. The highest weight patient was 100 kg and, therefore, received a single intravenous infusion of 1000 mg of Simponi.
Simponi DescriptionGolimumab is a human IgG1κ monoclonal antibody specific for human tumor necrosis factor alpha (TNFα) that exhibits multiple glycoforms with molecular masses of approximately 150 to 151 kilodaltons. Golimumab was created using genetically engineered mice immunized with human TNF, resulting in an antibody with human-derived antibody variable and constant regions. Golimumab is produced by a recombinant cell line cultured by continuous perfusion and is purified by a series of steps that includes measures to inactivate and remove viruses.
Simponi (golimumab) Injection is a preservative-free, sterile, clear to slightly opalescent, colorless to light yellow solution of the golimumab antibody supplied in a single-dose prefilled syringe (with a passive needle safety guard) or a single-dose prefilled autoinjector.
Each 0.5 mL prefilled syringe and autoinjector contains 50 mg golimumab, L-histidine and L-histidine monohydrochloride monohydrate (0.44 mg), polysorbate 80 (0.08 mg), sorbitol (20.5 mg) and Water for Injection. Each 1 mL prefilled syringe and autoinjector contains 100 mg golimumab, L-histidine and L-histidine monohydrochloride monohydrate (0.87 mg), polysorbate 80 (0.15 mg), sorbitol (41.0 mg) and Water for Injection. The pH is approximately 5.5.
Simponi - Clinical PharmacologyMechanism of ActionGolimumab is a human monoclonal antibody that binds to both the soluble and transmembrane bioactive forms of human TNFα. This interaction prevents the binding of TNFα to its receptors, thereby inhibiting the biological activity of TNFα (a cytokine protein). There was no evidence of the golimumab antibody binding to other TNF superfamily ligands; in particular, the golimumab antibody did not bind or neutralize human lymphotoxin. Golimumab did not lyse human monocytes expressing transmembrane TNF in the presence of complement or effector cells.
Elevated TNFα levels in the blood, synovium, and joints have been implicated in the pathophysiology of several chronic inflammatory diseases such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. TNFα is an important mediator of the articular inflammation that is characteristic of these diseases. The exact mechanism by which golimumab treats ulcerative colitis is unknown. Golimumab modulated the in vitro biological effects mediated by TNF in several bioassays, including the expression of adhesion proteins responsible for leukocyte infiltration (E-selectin, ICAM-1 and VCAM-1) and the secretion of proinflammatory cytokines (IL-6, IL-8, G-CSF and GM-CSF).
PharmacodynamicsIn clinical trials, decreases in C-reactive protein (CRP), interleukin (IL)-6, matrix metalloproteinase-3 (MMP-3), intercellular adhesion molecule (ICAM)-1 and vascular endothelial growth factor (VEGF) were observed following Simponi administration in patients with RA, PsA, and AS.
PharmacokineticsAbsorption
Following subcutaneous administration of Simponi to healthy subjects and patients with active RA, the median time to reach maximum serum concentrations (Tmax) ranged from 2 to 6 days. A subcutaneous injection of 50-mg Simponi to healthy subjects produced a mean ± standard deviation maximum serum concentration (Cmax) of 3.2 ± 1.4 mcg/mL.
By cross-trial comparisons of mean AUCinf values following an IV or subcutaneous administration of Simponi, the absolute bioavailability of subcutaneous Simponi was estimated to be approximately 53%.
Distribution
Following a single IV administration over the dose range of 0.1 to 10.0 mg/kg in patients with active RA, mean volume of distribution ranged from 58 to 126 mL/kg. The volume of distribution for Simponi indicates that Simponi is distributed primarily in the circulatory system with limited extravascular distribution.
Metabolism
The exact metabolic pathway of golimumab is unknown.
Elimination
Following a single IV administration over the dose range of 0.1 to 10.0 mg/kg in patients with active RA, mean systemic clearance of Simponi was estimated to be 4.9 to 6.7 mL/day/kg.
Median terminal half-life values were estimated to be approximately 2 weeks in healthy subjects and patients with active RA, PsA or AS.
Population PK analyses indicated that concomitant use of NSAIDs, oral corticosteroids, or sulfasalazine did not influence the apparent clearance of Simponi.
Patients who developed anti-golimumab antibodies generally had lower steady-state serum trough concentrations of Simponi.
Dose Linearity
Simponi exhibited dose-proportional pharmacokinetics (PK) in patients with active RA over the dose range of 0.1 to 10 mg/kg following a single intravenous (IV) dose. Following a single SC dose in healthy subjects, dose proportional pharmacokinetics were also observed over a dose range of 50 mg to 400 mg.
Single Dose Versus Multiple Doses
When 50-mg Simponi was administered subcutaneous to patients with RA, PsA, or AS every 4 weeks, serum concentrations appeared to reach steady state by Week 12. With concomitant use of methotrexate (MTX), treatment with 50-mg Simponi subcutaneous every 4 weeks resulted in a mean steady-state trough serum concentration of approximately 0.4–0.6 mcg/mL in patients with active RA, approximately 0.5 mcg/mL in patients with active PsA, and approximately 0.8 mcg/mL in patients with active AS. Patients with RA, PsA, and AS treated with Simponi 50 mg and MTX had approximately 52%, 36% and 21% higher mean steady-state trough concentrations of golimumab, respectively compared with those treated with Simponi 50 mg without MTX. The presence of MTX also decreased anti-golimumab antibody incidence from 7% to 2% [see Adverse Reactions (6.1)]. For RA, Simponi should be used with MTX. In the PsA and AS trials, the presence or absence of concomitant MTX did not appear to influence clinical efficacy and safety parameters [see Drug Interactions (7.1) and Clinical Studies (14.1)].
When induction doses of 200-mg and 100-mg Simponi at week 0 and 2, respectively, followed by maintenance doses of 100-mg Simponi every 4 weeks were administered subcutaneously in patients with UC, serum golimumab concentrations reached steady-state by week 8 after the first maintenance dose. Treatment with 100-mg Simponi subcutaneous every 4 weeks during maintenance resulted in a mean steady-state trough serum concentration of approximately 1.8 ± 1.1 mcg/mL.
Effect of Weight on Pharmacokinetics
Population PK analyses showed there was a trend toward higher apparent clearance of Simponi with increasing weight. Treatment with the recommended maintenance dose regimen of Simponi 100 mg in UC patients did not result in meaningful differences in clinical efficacy among different weight groups. Across the PsA and AS populations, no meaningful differences in clinical efficacy were observed among the subgroups by weight quartile. The RA trial in MTX-experienced and TNF-blocker-naïve patients (Trial RA-2) did show evidence of a reduction in clinical efficacy with increasing body weight, but this effect was observed for both tested doses of Simponi (50 mg and 100 mg). There is no need to adjust the dosage of Simponi based on a patient's weight.
Specific Populations
Population PK analyses suggested no PK differences between male and female patients after body-weight adjustment in the RA, PsA and UC trials. In the AS trial, female patients showed 13% higher apparent clearance than male patients after body-weight adjustment. Subgroup analysis based on gender showed that both female and male patients achieved clinically significant response at the proposed clinical dose. Dosage adjustment based on gender is not needed.
Population PK analyses indicated that PK parameters of Simponi were not influenced by age in adult patients. Patients with age ≥ 65 years had apparent clearance of Simponi similar to patients with age < 65 years. No ethnicity-related PK differences were observed between Caucasians and Asians, and there were too few patients of other races to assess for PK differences.
No formal trial of the effect of renal or hepatic impairment on the PK of golimumab was conducted.
Nonclinical ToxicologyCarcinogenesis, Mutagenesis, Impairment of FertilityLong-term animal studies of golimumab have not been conducted to evaluate its carcinogenic potential. Mutagenicity studies have not been conducted with golimumab. A fertility study conducted in mice using an analogous anti-mouse TNFα antibody administered by the intravenous route at doses up to 40 mg/kg once per week showed no impairment of fertility.
Clinical StudiesRheumatoid ArthritisThe efficacy and safety of Simponi were evaluated in 3 multicenter, randomized, double-blind, controlled trials (Trials RA-1, RA-2, and RA-3) in 1542 patients ≥ 18 years of age with moderately to severely active RA, diagnosed according to the American College of Rheumatology (ACR) criteria, for at least 3 months prior to administration of trial agent. Patients were required to have at least 4 swollen and 4 tender joints. Simponi was administered subcutaneously at doses of 50 mg or 100 mg every 4 weeks. Double-blinded controlled efficacy data were collected and analyzed through Week 24. Patients were allowed to continue stable doses of concomitant low dose corticosteroids (equivalent to ≤ 10 mg of prednisone a day) and/or NSAIDs and patients may have received oral MTX during the trials.
Trial RA-1 evaluated 445 patients who were previously treated (at least 8 to 12 weeks prior to administration of trial agent) with one or more doses of a biologic TNF blocker without a serious adverse reaction. Patients may have discontinued the biologic TNF blocker for a variety of reasons. Patients were randomized to receive placebo (N=150), Simponi 50 mg (N=147), or Simponi 100 mg (N=148). Patients were allowed to continue stable doses of concomitant MTX, sulfasalazine (SSZ), and/or hydroxychloroquine (HCQ) during the trial. The use of other DMARDs including cytotoxic agents or other biologics was prohibited.
Trial RA-2 evaluated 444 patients who had active RA despite a stable dose of at least 15 mg/week of MTX and who had not been previously treated with a biologic TNF blocker. Patients were randomized to receive background MTX (N=133), Simponi 50 mg + background MTX (N=89), Simponi 100 mg + background MTX (N=89), or Simponi 100 mg monotherapy (N=133). The use of other DMARDs including SSZ, HCQ, cytotoxic agents, or other biologics was prohibited.
Trial RA-3 evaluated 637 patients with active RA who were MTX naïve and had not previously been treated with a biologic TNF blocker. Patients were randomized to receive MTX (N=160), Simponi 50 mg + MTX (N=159), Simponi 100 mg + MTX (N=159), or Simponi 100 mg monotherapy (N=159). For patients receiving MTX, MTX was administered at a dose of 10 mg/week beginning at Week 0 and increased to 20 mg/week by Week 8. The use of other DMARDs including SSZ, HCQ, cytotoxic agents, or other biologics was prohibited.
The primary endpoint in Trial RA-1 and Trial RA-2 was the percentage of patients achieving an ACR 20 response at Week 14 and the primary endpoint in Trial RA-3 was the percentage of patients achieving an ACR 50 response at Week 24.
In Trials RA-1, RA-2, and RA-3, the median duration of RA disease was 9.4, 5.7, and 1.2 years and 99%, 75%, and 54% of the patients used at least one DMARD in the past, respectively. Approximately 77% and 57% of patients received concomitant NSAIDs and low dose corticosteroids, respectively, in the 3 pooled RA trials.
Clinical Response
In the 3 RA trials, a greater percentage of patients treated with the combination of Simponi and MTX achieved ACR responses at Week 14 (Trials RA-1 and RA-2) and Week 24 (Studies RA-1, RA-2, and RA-3) versus patients treated with the MTX alone. There was no clear evidence of improved ACR response with the higher Simponi dose group (100 mg) compared to the lower Simponi dose group (50 mg). In Trials RA-2 and RA-3, the Simponi monotherapy groups were not statistically different from the MTX monotherapy groups in ACR responses. Table 2 shows the proportion of patients with the ACR response for the Simponi 50-mg and control groups in Trials RA-1, RA-2, and RA-3. In the subset of patients who received Simponi in combination with MTX in Trial RA-1, the proportion of patients achieving ACR 20, 50 and 70 responses at Week 14 were 40%, 18%, and 12%, respectively, in the Simponi 50-mg + MTX group (N=101) compared with 17%, 6%, and 2%, respectively, in the placebo + MTX group (N=103). Table 3 shows the percent improvement in the components of the ACR response criteria for the Simponi 50-mg + MTX and MTX groups in Trial RA-2. The percentage of patients achieving ACR 20 responses by visit for Trial RA-2 is shown in Figure 1. ACR 20 responses were observed in 38% of patients in the Simponi 50-mg + MTX group at the first assessment (Week 4) after the initial Simponi administration.
|
Trial RA-1 Active RA previously treated with one or more doses of TNF blockers |
Trial RA-2 Active RA, despite MTX |
Trial RA-3 Active RA, MTX Naïve |
||||
|---|---|---|---|---|---|---|
| Placebo ± DMARDs† | Simponi 50 mg ± DMARDs† | Background MTX | Simponi 50 mg + Background MTX | MTX | Simponi 50 mg + MTX | |
| *Approximately 78% and 58% of the patients received concomitant NSAIDs and low dose corticosteroids (equivalent to ≤ 10 mg of prednisone a day), respectively, during the 3 pooled RA trials.†DMARDs in Trial RA-1 included MTX, HCQ, and/or SSZ (about 68%, 8%, and 5% of patients received MTX, HCQ, and SSZ, respectively).‡N reflects randomized patients.§NA = Not applicable, as data was not collected at Week 14 in Trial RA-3.¶Not significantly different from MTX monotherapy. | ||||||
| N‡ | 150 | 147 | 133 | 89 | 160 | 159 |
| ACR 20 | ||||||
| Week 14 | 18% | 35% | 33% | 55% | NA§ | NA§ |
| Week 24 | 16% | 31% | 28% | 60% | 49% | 62% |
| ACR 50 | ||||||
| Week 14 | 7% | 15% | 10% | 35% | NA§ | NA§ |
| Week 24 | 4% | 16% | 14% | 37% | 29% | 40% |
| ACR 70 | ||||||
| Week 14 | 2% | 10% | 4% | 13% | NA§ | NA§ |
| Week 24 | 2% | 9% | 5% | 20% | 16% | 24%¶ |
| Background MTX | Simponi 50 mg + Background MTX | |
|---|---|---|
| Note: Baseline values are medians. | ||
| *In Trial RA-2, about 70% and 85% of patients received concomitant low dose corticosteroids (equivalent to ≤ 10 mg of prednisone a day) and/or NSAIDs during the trials, respectively.†N reflects randomized patients; actual number of patients evaluable for each endpoint may vary. | ||
| N† | 133 | 89 |
| Number of swollen joints (0–66) | ||
| Baseline | 12 | 13 |
| Week 14 | 38% | 62% |
| Number of tender joints (0–68) | ||
| Baseline | 21 | 26 |
| Week 14 | 30% | 60% |
| Patient's assessment of pain (0–10) | ||
| Baseline | 5.7 | 6.1 |
| Week 14 | 18% | 55% |
| Patient's global assessment of disease activity (0–10) | ||
| Baseline | 5.3 | 6.0 |
| Week 14 | 15% | 45% |
| Physician's global assessment of disease activity (0–10) | ||
| Baseline | 5.7 | 6.1 |
| Week 14 | 35% | 55% |
| HAQ score (0–3) | ||
| Baseline | 1.25 | 1.38 |
| Week 14 | 10% | 29% |
| CRP (mg/dL) | ||
| Baseline | 0.8 | 1.0 |
| Week 14 | 2% | 44% |
| *The same patients may not have responded at each timepoint. |
| Figure 1: Trial RA-2 – Percentage of Patients Achieving ACR 20 Response by Visit: Randomized Patients* |
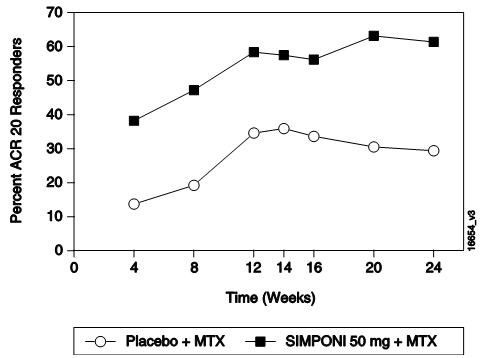
|
Physical Function Response in Patients with RA
In Trials RA-1 and RA-2, the Simponi 50-mg groups demonstrated a greater improvement compared to the control groups in the change in mean Health Assessment Questionnaire Disability Index (HAQ-DI) score from baseline to Week 24: 0.23 vs. 0.03 in RA-1, 0.47 vs. 0.13 in RA-2, respectively. Also in Trials RA-1 and RA-2, the Simponi 50-mg groups compared to the control groups had a greater proportion of HAQ responders (change from baseline > 0.22) at Week 24: 43% vs. 27%, 65% vs. 35%, respectively.
Psoriatic ArthritisThe safety and efficacy of Simponi were evaluated in a multicenter, randomized, double-blind, placebo-controlled trial in 405 adult patients with moderately to severely active PsA (≥ 3 swollen joints and ≥ 3 tender joints) despite NSAID or DMARD therapy (Trial PsA). Patients in this trial had a diagnosis of PsA for at least 6 months with a qualifying psoriatic skin lesion of at least 2 cm in diameter. Previous treatment with a biologic TNF blocker was not allowed. Patients were randomly assigned to placebo (N=113), Simponi 50 mg (N=146), or Simponi 100 mg (N=146) given subcutaneously every 4 weeks. Patients were allowed to receive stable doses of concomitant MTX (≤ 25 mg/week), low dose oral corticosteroids (equivalent to ≤ 10 mg of prednisone a day), and/or NSAIDs during the trial. The use of other DMARDs including SSZ, HCQ, cytotoxic agents, or other biologics was prohibited. The primary endpoint was the percentage of patients achieving ACR 20 response at Week 14. Placebo-controlled efficacy data were collected and analyzed through Week 24.
Patients with each subtype of PsA were enrolled, including polyarticular arthritis with no rheumatoid nodules (43%), asymmetric peripheral arthritis (30%), distal interphalangeal (DIP) joint arthritis (15%), spondylitis with peripheral arthritis (11%), and arthritis mutilans (1%). The median duration of PsA disease was 5.1 years, 78% of patients received at least one DMARD in the past, and approximately 48% of patients received MTX, and 16% received low dose oral steroids.
Clinical Response in Patients with PsA
Simponi ± MTX, compared with placebo ± MTX, resulted in significant improvement in signs and symptoms as demonstrated by the proportion of patients with an ACR 20 response at Week 14 in Trial PsA (see Table 4). There was no clear evidence of improved ACR response with the higher Simponi dose group (100 mg) compared to the lower Simponi dose group (50 mg). ACR responses observed in the Simponi-treated groups were similar in patients receiving and not receiving concomitant MTX. Similar ACR 20 responses at Week 14 were observed in patients with different PsA subtypes. However, the number of patients with arthritis mutilans was too small to allow meaningful assessment. Simponi 50-mg treatment also resulted in significantly greater improvement compared with placebo for each ACR component in Trial PsA (Table 5). Treatment with Simponi resulted in improvement in enthesitis and skin manifestations in patients with PsA. However, the safety and efficacy of Simponi in the treatment of patients with plaque psoriasis has not been established.
The percentage of patients achieving ACR 20 responses by visit for Trial PsA is shown in Figure 2. ACR 20 responses were observed in 31% of patients in the Simponi 50-mg + MTX group at the first assessment (Week 4) after the initial Simponi administration.
| Placebo ± MTX* | Simponi 50 mg ± MTX* | |
|---|---|---|
| Bold text indicates primary endpoint. | ||
| *In Trial PsA, about 48%, 16%, and 72% of the patients received stable doses of MTX (≤ 25 mg/week), low dose corticosteroids (equivalent to ≤ 10 mg of prednisone a day), and NSAIDs, respectively.†N reflects randomized patients. | ||
| N† | 113 | 146 |
| ACR 20 | ||
| Week 14 | 9% | 51% |
| Week 24 | 12% | 52% |
| ACR 50 | ||
| Week 14 | 2% | 30% |
| Week 24 | 4% | 32% |
| ACR 70 | ||
| Week 14 | 1% | 12% |
| Week 24 | 1% | 19% |
| Placebo ± MTX* | Simponi 50 mg ± MTX* | |
|---|---|---|
| Note: Baseline are median values. | ||
| *In Trial PsA, about 48%, 16%, and 78% of the patients received stable doses of MTX (≤ 25 mg/week), low dose corticosteroids (equivalent to ≤ 10 mg of prednisone a day), and NSAIDs, respectively.†N reflects randomized patients; actual number of patients evaluable for each endpoint may vary by timepoint. | ||
| N† | 113 | 146 |
| Number of swollen joints (0–66) | ||
| Baseline | 10.0 | 11.0 |
| Week 14 | 8% | 60% |
| Number of tender joints (0–68) | ||
| Baseline | 18.0 | 19.0 |
| Week 14 | 0% | 54% |
| Patient's assessment of pain (0–10) | ||
| Baseline | 5.4 | 5.8 |
| Week 14 | -1% | 48% |
| Patient's global assessment of disease activity (0–10) | ||
| Baseline | 5.2 | 5.2 |
| Week 14 | 2% | 49% |
| Physician's global assessment of disease activity (0–10) | ||
| Baseline | 5.2 | 5.4 |
| Week 14 | 7% | 59% |
| HAQ score (0–10) | ||
| Baseline | 1.0 | 1.0 |
| Week 14 | 0% | 28% |
| CRP (mg/dL) (0–10) | ||
| Baseline | 0.6 | 0.6 |
| Week 14 | 0% | 40% |
| *The same patients may not have responded at each timepoint. |
| Figure 2: Trial PsA – Percentage of ACR 20 PsA Responders by Visit: Randomized Patients* |
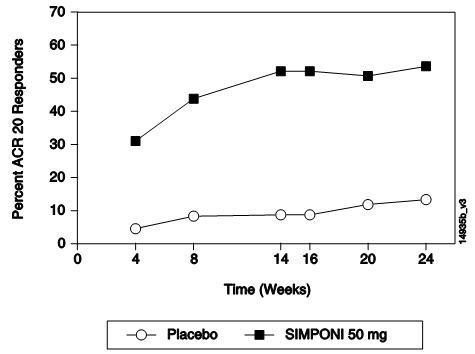
|
Physical Function Response in Patients with PsA
In Trial PsA, Simponi 50 mg demonstrated a greater improvement compared to placebo in the change in mean Health Assessment Questionnaire Disability Index (HAQ-DI) score from baseline to Week 24 (0.33 and -0.01, respectively). In addition, the Simponi 50-mg group compared to the placebo group had a greater proportion of HAQ responders (≥ 0.3 change from baseline) at Week 24: 43% vs. 22%, respectively.
Ankylosing SpondylitisThe safety and efficacy of Simponi were evaluated in a multicenter, randomized, double-blind, placebo-controlled trial in 356 adult patients with active ankylosing spondylitis according to modified New York criteria for at least 3 months (Trial AS). Patients had symptoms of active disease [defined as a Bath AS Disease Activity Index (BASDAI) ≥ 4 and VAS for total back pain of ≥ 4, on scales of 0 to 10 cm] despite current or previous NSAID therapy. Patients were excluded if they were previously treated with a biologic TNF blocker or if they had complete ankylosis of the spine. Patients were randomly assigned to placebo (N=78), Simponi 50 mg (N=138), or Simponi 100 mg (N=140) administered subcutaneously every 4 weeks. Patients were allowed to continue stable doses of concomitant MTX, sulfasalazine (SSZ), hydroxychloroquine (HCQ), low dose corticosteroids (equivalent to < 10 mg of prednisone a day), and/or NSAIDs during the trial. The use of other DMARDs including cytotoxic agents or other biologics was prohibited.
The primary endpoint was the percentage of patients achieving an ASsessment in Ankylosing Spondylitis (ASAS) 20 response at Week 14. Placebo-controlled efficacy data were collected and analyzed through Week 24.
In Trial AS, the median duration of AS disease was 5.6 years, median duration of inflammatory back pain was 12 years, 83% were HLA-B27 positive, 24% had prior joint surgery or procedure, and 55% received at least one DMARD in the past. During the trial, the use of concomitant DMARDs and/or NSAIDs was as follows: MTX (20%), SSZ (26%), HCQ (1%), low dose oral steroids (16%), and NSAIDs (90%).
Clinical Response in Patients with AS
In Trial AS, Simponi ± DMARDs treatment, compared with placebo ± DMARDs, resulted in a significant improvement in signs and symptoms as demonstrated by the proportion of patients with an ASAS 20 response at Week 14 (see Table 6). There was no clear evidence of improved ASAS response with the higher Simponi dose group (100 mg) compared to the lower Simponi dose group (50 mg). Table 7 shows the percent improvement in the components of the ASAS response criteria for the Simponi 50 mg ± DMARDs and placebo ± DMARDs groups in Trial AS.
The percentage of patients achieving ASAS 20 responses by visit for Trial AS is shown in Figure 3. ASAS 20 responses were observed in 48% of patients in the Simponi 50-mg + MTX group at the first assessment (Week 4) after the initial Simponi administration.
| Placebo ± DMARDs* | Simponi 50 mg ± DMARDs* | |
|---|---|---|
| Bold text indicates primary endpoint. | ||
| *During the trial, the concomitant use of stable doses of DMARDS was as follows: MTX (21%), SSZ (25%), and HCQ (1%). About 16% and 89% of patients received stable doses of low dose oral steroids and NSAIDs during the trial, respectively.†N reflects randomized patients. | ||
| N† | 78 | 138 |
| Responders, % of patients | ||
| ASAS 20 | ||
| Week 14 | 22% | 59% |
| Week 24 | 23% | 56% |
| ASAS 40 | ||
| Week 14 | 15% | 45% |
| Week 24 | 15% | 44% |
| Placebo ± DMARDs* | Simponi 50 mg ± DMARDs* | |
|---|---|---|
| *During the trial, the concomitant use of stable doses of DMARDS was as follows: MTX (21%), SSZ (25%), and HCQ (1%). About 16% and 89% of patients received stable doses of low dose oral steroids and NSAIDs during the trial, respectively.†N reflects randomized patients.‡BASFI is Bath Ankylosing Spondylitis Functional Index.§Inflammation is the mean of 2 patient-reported stiffness self-assessments in the Bath AS Disease Activity Index (BASDAI). | ||
| N† | 78 | 138 |
| ASAS components | ||
| Patient global assessment (0–10) | ||
| Baseline | 7.2 | 7.0 |
| Week 14 | 13% | 47% |
| Total back pain (0–10) | ||
| Baseline | 7.6 | 7.5 |
| Week 14 | 9% | 50% |
| BASFI (0–10)‡ | ||
| Baseline | 4.9 | 5.0 |
| Week 14 | -3% | 37% |
| Inflammation (0–10)§ | ||
| Baseline | 7.1 | 7.1 |
| Week 14 | 6% | 59% |
| *The same patients may not have responded at each timepoint. |
| Figure 3: Trial AS – Percentage of AS Patients Achieving ASAS 20 Response by Visit: Randomized Patients* |
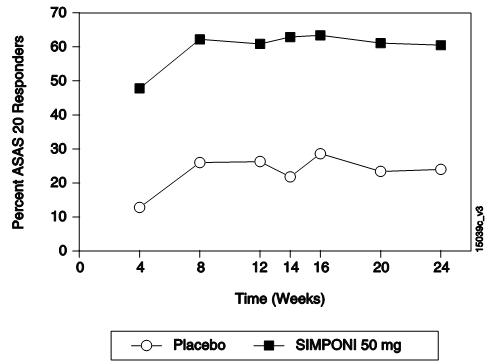
|
The safety and efficacy of Simponi were evaluated in 2 multicenter, randomized, double-blind, placebo-controlled clinical trials in patients ≥ 18 years of age (Trials UC-1 and UC-2).
Trial UC-1 was an induction trial conducted in patients with moderately to severely active ulcerative colitis (UC), defined as a Mayo score of 6 to 12 [the Mayo score ranges from 0 to 12 and has 4 subscales that are each scored from 0 (normal) to 3 (most severe): stool frequency, rectal bleeding, findings on endoscopy, and physician global assessment]. At baseline, subjects also had an endoscopy subscore of 2 or 3 on a 3-point scale (an endoscopy score of 2 is defined by marked erythema, absent vascular pattern, friability, erosions; and a score of 3 is defined by spontaneous bleeding, ulceration). Patients were corticosteroid dependent (i.e., an inability to successfully taper corticosteroids without a return of the symptoms of UC) or had an inadequate response to or had failed to tolerate at least one of the following therapies: oral aminosalicylates, oral corticosteroids, azathioprine, or 6-mercaptopurine.
Trial UC-1 was divided into 2 parts. In Part 1 (dose finding), patients were randomized to one of 4 treatment groups: 400 mg Simponi administered subcutaneously (SC) at Week 0 and 200 mg at Week 2 (400/200 mg), 200-mg Simponi SC at Week 0 and 100 mg at Week 2 (200/100 mg), 100-mg Simponi SC at Week 0 and 50 mg at Week 2 (100/50 mg), or placebo SC at Weeks 0 and 2. In Part 2 (dose confirming), efficacy was evaluated in 761 patients who were randomized to receive either 400 mg Simponi SC at Week 0 and 200 mg at Week 2, 200-mg Simponi SC at Week 0 and 100 mg at Week 2, or placebo SC at Weeks 0 and 2. Simponi 100/50-mg SC was not evaluated in Part 2; its safety and effectiveness has not been established in UC. Concomitant stable doses of oral aminosalicylates (5-ASA), oral corticosteroids (less than 40 mg/day), azathioprine (AZA), 6-mercaptopurine (6-MP), and/or methotrexate (MTX) were permitted. Patients who received previous TNF inhibitors were excluded. The primary endpoint was the percent of patients in clinical response at Week 6, defined as a decrease from baseline in the Mayo score by ≥ 30% and ≥ 3 points, accompanied by a decrease in the rectal bleeding subscore of ≥ 1 or a rectal bleeding subscore of 0 (no blood seen) or 1 (streaks of blood with stool less than half the time).
Trial UC-2 was a randomized-withdrawal maintenance trial that evaluated 456 patients who achieved clinical response with Simponi induction and tolerated Simponi treatment. Patients were randomized to receive Simponi 50 mg, Simponi 100 mg or placebo administered subcutaneously every 4 weeks. Concomitant stable doses of oral aminosalicylates, azathioprine, 6-mercaptopurine, and/or methotrexate were permitted. Corticosteroids were to be tapered at the start of the maintenance trial. The primary endpoint was the percent of patients maintaining clinical response through Week 54.
Clinical Response, Clinical Remission and Improvement of Endoscopic Appearance of the Mucosa
In Trial UC-1, a greater proportion of patients achieved clinical response, clinical remission and had improvement of endoscopic appearance of the mucosa at Week 6 in the Simponi 200/100-mg group compared with the placebo group. The Simponi 400/200-mg group did not demonstrate additional clinical benefit over the Simponi 200/100-mg group. Clinical response was defined as a decrease from baseline in the Mayo score of ≥ 30% and ≥ 3 points, accompanied by a decrease in the rectal bleeding subscore of ≥ 1 or a rectal bleeding subscore of 0 or 1. Clinical remission was defined as a Mayo score ≤ 2 points, with no individual subscore > 1. Improvement of endoscopic appearance of the mucosa was defined as a Mayo endoscopy subscore of 0 (normal or inactive disease) or 1 (erythema, decreased vascular pattern, mild friability).
In Trial UC-2, a greater proportion of patients maintained clinical response through Week 54 in the Simponi 100-mg group compared with the placebo group. In Trial UC-2, Simponi-treated patients in clinical response (which included the subset of patients in clinical remission) in Trial UC-1, were again assessed for clinical remission at Week 30 and Week 54. A greater proportion of patients had clinical remission at both Weeks 30 and 54 without demonstrating a loss of response at any time point through Week 54 in the Simponi 100-mg group compared with the placebo group.
These results are shown in Table 8 below.
| *Patients who had a prohibited change in concomitant UC medication, an ostomy or colectomy, discontinued trial agent due to lack of therapeutic effect, or a dose adjustment in Trial UC-2 were considered not to be in clinical response, clinical remission or have an improvement in endoscopic appearance of the mucosa from the time of the event onward.†p<0.0001;‡p=0.0014;§Results in Trial UC-2 are based on patients who were in clinical response to Simponi at trial entry.¶Patients were assessed for UC disease activity by partial Mayo score every 4 weeks (loss of response was confirmed by endoscopy). Therefore, a patient who maintained clinical response was in response at each evaluation through Week 54.#p<0.001;ÞA patient had to be in remission at both Weeks 30 and 54 (without demonstrating a loss of response at any time point through Week 54) to achieve sustained remission.ßp=0.004 | |||
| Trial UC-1 (6-Week Induction Trial) | |||
|
Placebo N=251 |
Simponi 200/100 mg N=253 |
Treatment difference (95% C.I.) |
|
| Clinical response* at Week 6 | 30% | 51% |
21% (12%, 29%)† |
| Clinical remission* at Week 6 | 6% | 18% |
11% (6%, 17%)† |
| Improvement of endoscopic appearance of the mucosa at Week 6* | 29% | 42% |
14% (5%, 22%)‡ |
| Trial UC-2 (54-Week Maintenance Trial)§ | |||
|
Placebo N=154 |
Simponi 100 mg N=151 |
Treatment difference (95% C.I.) |
|
| Clinical response* through Week 54¶ | 31% | 50% |
19% (8%, 29%)# |
| Clinical remission* at both Week 30 and Week 54Þ | 16% | 28% |
12% (3%, 21%)ß |
1. SEER [database online]. US Population Data – 1969–2004. Bethesda, MD: National Cancer Institute. Release date: January 3, 2007. Available at: http//seer.cancer.gov/popdata/.
How Supplied/Storage and HandlingSimponi (golimumab) Injection is a preservative-free, sterile, clear to slightly opalescent, colorless to light yellow solution for subcutaneous use in a single-dose prefilled autoinjector (contains a prefilled glass syringe) or single-dose prefilled glass syringe. The Type 1 glass syringe has a coated stopper. The fixed stainless steel needle (5 bevel, 27G, ½ inch) is covered with a needle shield to prevent leakage of the solution through the needle and to protect the needle during handling prior to subcutaneous administration. The needle shield is made of a dry natural rubber containing latex.
| 50 mg/0.5 mL single-dose prefilled syringe | 1 pack | NDC 57894-070-01 |
| 100 mg/mL single-dose prefilled syringe | 1 pack | NDC 57894-071-01 |
| 50 mg/0.5 mL single-dose prefilled SmartJect® autoinjector | 1 pack | NDC 57894-070-02 |
| 100 mg/mL single-dose prefilled SmartJect® autoinjector | 1 pack | NDC 57894-071-02 |
Storage and Handling
Refrigerate Simponi at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light until the time of use. Do not freeze. Do not shake. Do not use Simponi beyond the expiration date (EXP) on the carton or the expiration date on the prefilled syringe (observed through the viewing window) or the prefilled SmartJect autoinjector.
If needed, Simponi may be stored at room temperature up to 77°F (25°C) for a maximum single period of 30 days in the original carton to protect from light. Once a syringe or autoinjector has been stored at room temperature, do not return the product to the refrigerator. If not used within 30 days at room temperature, discard Simponi.
Patient Counseling InformationSee FDA-approved patient labeling (Medication Guide and Instructions for Use)
Patients should be advised of the potential benefits and risks of Simponi. Physicians should instruct their patients to read the Medication Guide before starting Simponi therapy and to read it each time the prescription is renewed.
Infections
Inform patients that Simponi may lower the ability of their immune system to fight infections. Instruct the patient of the importance of contacting their doctor if they develop any symptoms of infection, including tuberculosis, invasive fungal infections, and hepatitis B reactivation.
Malignancies
Patients should be counseled about the risk of lymphoma and other malignancies while receiving Simponi.
Allergic Reactions
Advise latex-sensitive patients that the needle cover on the prefilled syringe as well as the prefilled syringe in the prefilled SmartJect autoinjector contains dry natural rubber (a derivative of latex).
Other Medical Conditions
Advise patients to report any signs of new or worsening medical conditions such as congestive heart failure, demyelinating disorders, autoimmune diseases, liver disease, cytopenias, or psoriasis.
Instructions for Safe Administration
The first self-injection should be performed under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer Simponi, he/she should be instructed in injection techniques and their ability to inject subcutaneously should be assessed to ensure the proper administration of Simponi.
Advise the patient to read the FDA-approved Instructions for Use and provide the following instructions to patients:
Prior to use, remove the prefilled syringe or the prefilled SmartJect autoinjector from the refrigerator and allow Simponi to sit at room temperature outside of the carton for at least 30 minutes and out of the reach of children.Do not warm Simponi in any other way. For example, do not warm Simponi in a microwave or in hot water.Do not remove the prefilled syringe needle cover or SmartJect autoinjector cap while allowing Simponi to reach room temperature. Remove these immediately before injection.Do not pull the autoinjector away from the skin until you hear a first "click" sound and then a second "click" sound (the injection is finished and the needle is pulled back). It usually takes about 3 to 6 seconds but may take up to 15 seconds for you to hear the second "click" after the first "click". If the autoinjector is pulled away from the skin before the injection is completed, a full dose of Simponi may not be administered.A puncture-resistant container for disposal of needles and syringes should be used. Patients or caregivers should be instructed in the technique of proper syringe and needle disposal, and be advised not to reuse these items.
Manufactured by:
Janssen Biotech, Inc.
Horsham, PA 19044
US License No. 1864
© 2014 Janssen Pharmaceutical Companies
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: May 2018 | ||
|
MEDICATION GUIDE Simponi® (SIM-po-nee) (golimumab) injection, for subcutaneous use |
|||
|
What is the most important information I should know about Simponi? Simponi is a medicine that affects your immune system. Simponi can lower the ability of your immune system to fight infections. Some people have serious infections while taking Simponi, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that spread throughout their body. Some people have died from these serious infections.Your doctor should test you for TB and hepatitis B before starting Simponi.Your doctor should monitor you closely for signs and symptoms of TB during treatment with Simponi. You should not start taking Simponi if you have any kind of infection unless your doctor says it is okay. Before starting Simponi, tell your doctor if you: think you have an infection or have symptoms of an infection such as: |
|||
| fever, sweat, or chillsmuscle achescoughshortness of breathblood in phlegmweight loss | warm, red, or painful skin or sores on your bodydiarrhea or stomach painburning when you urinate or urinate more often than normalfeel very tired | ||
|
are being treated for an infection.get a lot of infections or have infections that keep coming back.have diabetes, HIV, or a weak immune system. People with these conditions have a higher chance for infections.have TB, or have been in close contact with someone with TB.live, have lived, or traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance for getting certain kinds of fungal infections (histoplasmosis, coccidioidomycosis, blastomycosis). These infections may happen or become more severe if you use Simponi. Ask your doctor if you do not know if you have lived in an area where these infections are common.have or have had hepatitis B.use the medicine ORENCIA (abatacept), KINERET (anakinra), ACTEMRA (tocilizumab) or RITUXAN (rituximab).After starting Simponi, call your doctor right away if you have any symptoms of an infection. Simponi can make you more likely to get infections or make worse any infection that you have. CancerFor children and adults taking TNF-blocker medicines, including Simponi, the chances of getting cancer may increase.There have been cases of unusual cancers in children and teenage patients taking TNF-blocking agents.People with inflammatory diseases including rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, especially those with very active disease, may be more likely to get lymphoma.Some people receiving medicines that are like Simponi, called TNF blockers, developed a rare type of cancer called hepatosplenic T cell lymphoma. This type of cancer often results in death. Most of these people were male teenagers or young men. Also, most people were being treated for Crohn's disease or ulcerative colitis with a TNF blocker and another medicine called azathioprine or 6 mercaptopurine (6-MP).Some people treated with Simponi have developed certain kinds of skin cancer. If any changes in the appearance of your skin or growths on your skin occur during or after your treatment with Simponi, tell your doctor.You should see your doctor periodically for skin examinations, especially if you have a history of skin cancer. |
|||
|
What is Simponi? Simponi is a prescription medicine called a Tumor Necrosis Factor (TNF) blocker. Simponi is used in adults:with the medicine methotrexate to treat moderately to severely active rheumatoid arthritis (RA)to treat active psoriatic arthritis (PsA) alone or with methotrexateto treat active ankylosing spondylitis (AS)with moderately to severely active ulcerative colitis (UC) when certain other UC medicines have not worked well enough or cannot be tolerated, or if it is necessary to continue taking steroid medicines:to begin helping some of your symptoms.in people who respond to Simponi, to get their UC under control (induce remission) and keep UC under control (sustain remission).to begin to improve the way the lining of your large intestine looks to your doctor during colonoscopy. You may continue to use other medicines that help treat your condition while taking Simponi, such as non-steroidal anti-inflammatory drugs (NSAIDs) and prescription steroids, as recommended by your doctor. It is not known if Simponi is safe and effective in children under 18 years of age. |
|||
|
What should I tell my doctor before starting treatment with Simponi? Simponi may not be right for you. See "What is the most important information I should know about Simponi?" Before starting Simponi, tell your doctor about all your medical conditions, including if you:have an infection.have or have had lymphoma or any other type of cancer.have or had heart failure.have or have had a condition that affects your nervous system, such as multiple sclerosis or Guillain-Barré syndrome.have recently received or are scheduled to receive a vaccine. People taking Simponi should not receive live vaccines or treatment with a weakened bacteria (such as BCG for bladder cancer). People taking Simponi can receive non-live vaccines.have a baby and you were using Simponi during your pregnancy. Tell your baby's doctor before your baby receives any vaccine. Your baby may have an increased chance of getting an infection for up to 6 months after birth.are allergic to rubber or latex. The needle cover on the prefilled syringe and SmartJect autoinjector contains dry natural rubber.are pregnant or planning to become pregnant. It is not known if Simponi will harm your unborn baby.are breastfeeding or plan to breastfeed. You and your doctor should decide if you will take Simponi or breastfeed.Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially, tell your doctor if you:use ORENCIA (abatacept) or KINERET (anakinra). You should not take Simponi while you are also taking ORENCIA (abatacept) or KINERET (anakinra).use other TNF-blocker medicines, including REMICADE (infliximab), HUMIRA (adalimumab), ENBREL (etanercept), or CIMZIA (certolizumab pegol).receive RITUXAN (rituximab) or ACTEMRA (tocilizumab).
Ask your doctor if you are not sure if your medicine is one listed above. |
|||
| How should I use Simponi?Simponi is given as an injection under the skin (subcutaneous injection).If your doctor decides that you or a caregiver may be able to give your injections of Simponi at home, you should receive training on the right way to prepare and inject Simponi. Do not try to inject Simponi yourself until you have been shown the right way to give the injections by your doctor or nurse.Use Simponi exactly as prescribed by your doctor. Your doctor will tell you how much Simponi to inject and when to inject it depending on your medical condition.Simponi comes in a prefilled syringe or SmartJect autoinjector. Your doctor will prescribe the type that is best for you.See the detailed Instructions for Use that comes with your Simponi for information about the right way to prepare and give your Simponi injections at home.Do not miss any doses of Simponi. If you forget to use Simponi, inject your dose as soon as you remember. Then, take your next dose at your regular scheduled time. In case you are not sure when to inject Simponi, call your doctor or pharmacist. | |||
|
What are the possible side effects of Simponi? Simponi can cause serious side effects, including: See "What is the most important information I should know about Simponi?" Serious Infections.Some patients have an increased chance of getting serious infections while receiving Simponi. These serious infections include TB and infections caused by viruses, fungi, or bacteria that have spread throughout the body. Some patients die from these infections. If you get an infection while receiving treatment with Simponi your doctor will treat your infection and may need to stop your Simponi treatment. Tell your doctor right away if you have any of the following signs of an infection while taking or after taking Simponi: |
|||
| a feverfeel very tiredhave a cough | have flu-like symptomswarm, red, or painful skin | ||
| Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with Simponi and during treatment with Simponi. Even if your TB test is negative your doctor should carefully monitor you for TB infections while you are taking Simponi. People who had a negative TB skin test before receiving Simponi have developed active TB. Tell your doctor if you have any of the following symptoms while taking or after taking Simponi: | |||
| cough that does not go awaylow grade fever | weight lossloss of body fat and muscle (wasting) | ||
| Hepatitis B infection in people who carry the virus in their blood.If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use Simponi. Your doctor should do blood tests before you start treatment with Simponi and while you are using Simponi. Tell your doctor if you have any of the following symptoms of a possible hepatitis B infection: | |||
| feel very tireddark urineskin or eyes look yellowlittle or no appetitevomitingmuscle aches | clay-colored bowel movementsfeverschillsstomach discomfortskin rash | ||
| Heart failure, including new heart failure or worsening of heart failure that you already have, can happen in people who use TNF-blocker medicines including Simponi. If you develop new or worsening heart failure with Simponi, you may need to be treated in a hospital, and it may result in death.If you have heart failure before starting Simponi, your condition should be watched closely during treatment with Simponi.Call your doctor right away if you get new or worsening symptoms of heart failure during treatment with Simponi (such as shortness of breath or swelling of your lower legs or feet, or sudden weight gain). | |||
|
Nervous System Problems. Rarely, people using TNF-blocker medicines, including Simponi, have nervous system problems such as multiple sclerosis or Guillain-Barré syndrome. Tell your doctor right away if you get any of these symptoms: |
|||
| vision changesweakness in your arms or legs | numbness or tingling in any part of your body | ||
|
Immune System Problems. Rarely, people using TNF-blocker medicines have developed symptoms that are like the symptoms of Lupus. Tell your doctor if you have any of these symptoms: |
|||
| a rash on your cheeks or other parts of the bodysensitivity to the sunnew joint or muscle pains | becoming very tiredchest pain or shortness of breathswelling of the feet, ankles, or legs | ||
|
Liver Problems. Liver problems can happen in people who use TNF-blocker medicines, including Simponi. These problems can lead to liver failure and death. Call your doctor right away if you have any of these symptoms: |
|||
| feel very tiredskin or eyes look yellow | poor appetite or vomitingpain on the right side of your stomach (abdomen) | ||
|
Blood Problems. Low blood counts have been seen with Simponi. Your body may not make enough blood cells that help fight infections or help stop bleeding. Symptoms include fever, bruising or bleeding easily, or looking pale. Your doctor will check your blood counts before and during treatment with Simponi. |
|||
|
Allergic Reactions. Allergic reactions can happen in people who use TNF-blocker medicines, including Simponi. Some reactions may be serious and can be life-threatening. Some of these reactions can happen after receiving your first dose of Simponi. Call your doctor right away if you have any of these symptoms of an allergic reaction: |
|||
| hivesswollen face | breathing troublechest pain | ||
|
The most common side effects of Simponi include: upper respiratory infection (runny nose, sore throat, and hoarseness or laryngitis)reaction at the site of injection (redness, swelling, itching, pain, bruising, or tingling)viral infections such as flu and oral cold sores |
|||
|
Psoriasis. Some people using Simponi had new psoriasis or worsening of psoriasis they already had. Tell your doctor if you develop red scaly patches or raised bumps that are filled with pus. Your doctor may decide to stop your treatment with Simponi. These are not all of the possible side effects of Simponi. Tell your doctor about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
How should I store Simponi? Refrigerate Simponi at 36°F to 46°F (2°C to 8°C).If needed, you may store Simponi at room temperature up to 77°F (25°C) for one period of time up to 30 days.Write the date of that you remove Simponi from the refrigerator on the carton.If Simponi has reached room temperature, do not put it back in the refrigerator.Throw away Simponi if it has been kept at room temperature for 30 days and has not been used.Do not freeze Simponi.Keep Simponi in the original carton to protect it from light when not being used.Do not shake Simponi.Do not use Simponi after the expiration date on the carton or on the prefilled syringe or SmartJect autoinjector.Keep Simponi and all medicines out of the reach of children. |
|||
|
General information about the safe and effective use of Simponi.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Simponi for a condition for which it was not prescribed. Do not give Simponi to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about Simponi. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about Simponi that is written for health professionals. For more information go to www.Simponi.com or call 1-800-JANSSEN (1-800-526-7736). |
|||
|
What are the ingredients in Simponi?
Active ingredient: golimumab. Inactive ingredients: L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, sorbitol, and water for injection. Simponi does not contain preservatives. Manufactured by: Janssen Biotech, Inc. Horsham, PA 19044 US License No. 1864 © 2014 Janssen Pharmaceutical Companies |
|||
Simponi® (SIM-po-nee)
(golimumab)
SmartJect® autoinjector
Important
If your doctor decides that you or a caregiver may be able to give your Simponi injections at home, you should receive training on the right way to prepare and inject Simponi using SmartJect.
Do not try to inject Simponi yourself until you have been shown the right way to give the injections by your doctor or nurse.
Please read this Instructions for Use before using Simponi SmartJect and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.

|
Store Simponi in the refrigerator at 36° to 46°F (2° to 8°C). |
If needed, store Simponi at room temperature, up to 77°F (25°C) for one period of time up to 30 days. Do not return it to the refrigerator. Throw away (dispose of) if not used within 30 days at room temperature.
Do not freeze SmartJect.
Do not shake SmartJect.
Keep Simponi in the original carton to protect from light before use.
Keep Simponi and all medicines out of the reach of children.
Your SmartJect at-a-glance
1. Prepare for your injection
|
Take out SmartJect Take SmartJect out of the refrigerator and remove it from the carton. Place on a flat surface out of reach of children. |
SmartJect should sit at room temperature for at least 30 minutes to ensure proper injection. Do not warm any other way. Do not remove the cap yet.
|
Inspect SmartJect Check the expiration date ('EXP') on the back of SmartJect. Do not use Simponi SmartJect if the expiration date has passed. Call your doctor or pharmacist for a refill. Check the security seal on the cap. Do not inject if the seal is broken. |
|
Gather supplies While SmartJect sits at room temperature for 30 minutes, gather your supplies: 1 Alcohol swab1 Cotton ball or gauze pad1 Sharps container (See Step 3)
Check liquid in the SmartJect After 30 minutes, check the liquid in the viewing window. It should be clear to slightly yellow and may contain tiny white or clear particles. It is also normal to see a small air bubble. Do not inject if the liquid is cloudy or discolored, or has large particles. |
|
Choose injection site Select from the following areas for your injection: Front of thighs (recommended)Lower abdomen (do not use the 2-inch area around your navel (belly-button))Back of upper arms (if a caregiver is giving you the injection)Choose a different site within your preferred area for each injection. Do not inject into skin that is tender, bruised, red, scaly or hard. Do not inject into areas with scars or stretch marks. |
Clean injection site Wipe your chosen injection site with an alcohol swab and allow it to dry. Do not touch, fan or blow on the injection site after you have cleaned it. |
| 2. Inject Simponi using SmartJect | ||
|
Remove cap Twist the cap to break the security seal, then pull it straight off. Dispose of the cap right away. It is important to inject within 5 minutes of removing the cap. Do not put the cap back on, this may damage the hidden needle. Do not inject if SmartJect is dropped without the cap on. |
Position Hold SmartJect comfortably and position it straight onto your skin, as shown. Make sure the green safety sleeve is flat against your skin and that your injection site is as flat as possible. Do not touch or press the button while positioning the SmartJect onto your skin. |
Push firmly Push SmartJect firmly against your skin so the green safety sleeve slides into the clear cover. Do not touch or press the button while pushing SmartJect against your skin.
The green safety sleeve helps prevent accidental injections. You will not be able to press the button to start your injection until SmartJect is pushed firmly enough against your skin for the green safety sleeve to slide into the clear cover. |
|
Press button and wait Keep holding SmartJect firmly against your skin. Use your other hand to press the raised part of the button to start your injection. You will hear a loud 1st 'click' as you press the button. This is normal, the medication is just beginning to be delivered. You may or may not feel a needle prick. Do not lift SmartJect up yet! This may result in loss of medication. After 1 press of the button, you do not need to keep pressure on the button. Wait for the 2nd 'click' which means your injection is complete. |
Listen for 2nd 'click' Keep holding SmartJect firmly against your skin until you hear the 2nd 'click' (3–15 seconds). The 2nd 'click' means the injection is complete and you can lift SmartJect from your skin. If you have trouble hearing the 'clicks', count to 15 after pressing the button, then lift the SmartJect off your skin. |
Check the viewing window After lifting SmartJect from your skin, look for the yellow indicator in the viewing window to confirm SmartJect worked properly. The yellow indicator will fill about half of the viewing window. If you do not see the yellow indicator, call 800-JANSSEN (800-526-7736). Do not administer a second dose without speaking to your doctor. |
| 3. After your injection | |
|
Dispose of your SmartJect Put your used SmartJect in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash. Do not recycle your used sharps disposal container. For more information, see "Helpful tips".
Check injection site There may be a small amount of blood or liquid at the injection site. |
Hold pressure over your skin with a cotton ball or gauze pad until any bleeding stops. Do not rub the injection site. If needed, cover injection site with a bandage. Your injection is now complete! |
|
|
|
| Call your doctor to talk about any questions you may have. For additional assistance or to share your feedback call 800-JANSSEN (800-526-7736). | |
|
Helpful tips If you are having difficulty injecting: ✓Make sure the cap is removed.✓Make sure SmartJect is pushed firmly against your skin. |
✓Make sure you are pressing the raised part of button.✓Try pressing the button a little harder.✓Try a different injection site.
If you are pinching the skin to inject: |
Use 1 hand to both position SmartJect against the skin and press the button.
|
|
Additional disposal information: If you do not have an FDA-cleared sharps disposal container, you may use a household container that is: • made of a heavy-duty plastic • can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out • upright and stable during use • leak-resistant |
• properly labeled to warn of hazardous waste inside the container When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. |
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: www.fda.gov/safesharpsdisposal |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Janssen Biotech, Inc.
Horsham, PA 19044
US License No. 1864
Revised: May 2018
Instructions for Use
Simponi®
(SIM-po-nee)
(golimumab)
Prefilled Syringe


Important
Simponi comes as a single-dose prefilled syringe containing one 50 mg or one 100 mg dose. Each Simponi prefilled syringe can only be used one time. Throw away (dispose of) the used prefilled syringe (See Step 3) after one dose, even if there is medicine left in it. Do not reuse your Simponi prefilled syringe.
If your healthcare provider decides that you or a caregiver may be able to give your injections of Simponi at home, you should receive training on the right way to prepare and inject Simponi using the prefilled syringe before attempting to inject. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider.
Read this Instructions for Use before using your Simponi prefilled syringe and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
The Simponi prefilled syringe is intended for injection under the skin, not into the muscle or vein. After injection, the needle will retract into the body of the device and lock into place.
 Storage information
Storage information
Store Simponi in the refrigerator at 36° to 46°F (2° to 8°C).
If needed, store Simponi at room temperature, up to 77°F (25°C) for one period of time up to 30 days. Do not return it to the refrigerator. Throw away if not used within 30 days at room temperature.
Do not freeze Simponi prefilled syringe.
Do not shake Simponi prefilled syringe.
Keep Simponi prefilled syringe in the original carton to protect from light before use.
Keep Simponi prefilled syringe and all medicines out of the reach of children.
Prefilled syringe parts
Before use
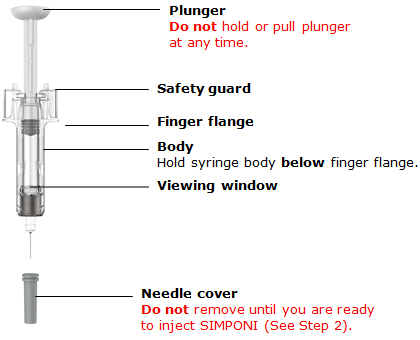
After use
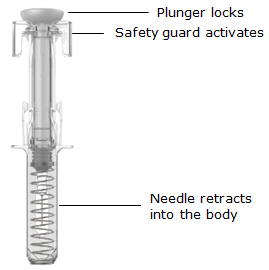
| You will need these supplies:1 Simponi prefilled syringe |
| Not provided in the Simponi prefilled syringe carton:1 Alcohol swab1 Cotton ball or gauze pad1 Adhesive bandage1 Sharps container (See Step 3) |

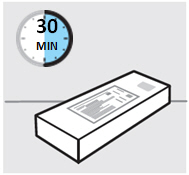
Inspect carton
Remove your Simponi prefilled syringe carton from the refrigerator.
Remove the prefilled syringe from the carton and let it sit on a flat surface at room temperature for at least 30 minutes before use.
Do not warm the prefilled syringe any other way.
Check the expiration date ('EXP') on the back panel of the carton and on the prefilled syringe (through the viewing window).
Do not use your prefilled syringe if the expiration date has passed.
Do not inject Simponi if the perforations on the carton are broken. Call your healthcare provider or pharmacist for a refill.
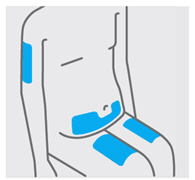
Choose injection site
Select from the following areas for your injection:
Front of thighs (recommended)Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)Back of upper arms (only if someone else is giving you the injection)Choose a different site within your preferred area for each injection.
Do not inject into skin that is tender, bruised, red, hard, thick or scaly.
Do not inject into areas with scars or stretch marks.
Clean injection site
Wash your hands well with soap and warm water.
Wipe your chosen injection site with an alcohol swab and allow it to dry.
Do not touch, fan, or blow on the injection site after you have cleaned it.
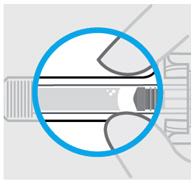
Inspect liquid
Check the Simponi prefilled syringe liquid in the viewing window. It should be clear to slightly yellow and may contain tiny white or clear particles. You may also see one or more air bubbles. This is normal.
Do not inject if the liquid is cloudy or discolored, or has large particles. Call your healthcare provider or pharmacist for a refill.

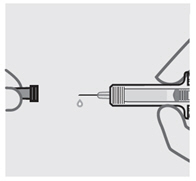
Remove needle cover
Hold your prefilled syringe by the body and pull needle cover straight off. It is normal to see a drop of liquid.
Inject Simponi within 5 minutes of removing the needle cover.
Do not put needle cover back on, as this may damage the needle or cause a needle stick injury.
Do not touch needle or let it touch any surface.
Do not use a Simponi prefilled syringe if it is dropped. Call your healthcare provider or pharmacist for a refill.
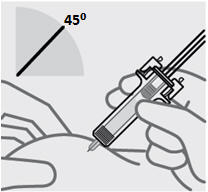
Position fingers and insert needle
Place your thumb, index and middle fingers directly under the finger flange, as shown.
Do not touch plunger or area above finger flange as this may cause the needle safety device to activate.
Use your other hand to pinch skin at the injection site. Position syringe at about a 45 degree angle to the skin.
It is important to pinch enough skin to inject under the skin and not into the muscle.
Insert needle with a quick, dart-like motion.
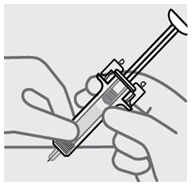
Release pinch and reposition hand
Use your free hand to grasp the body of the prefilled syringe.
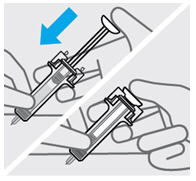
Press plunger
Place thumb from the opposite hand on the plunger and press the plunger all the way down until it stops.
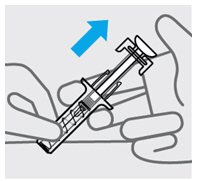
Release pressure from plunger
The safety guard will cover the needle and lock into place, removing the needle from your skin.

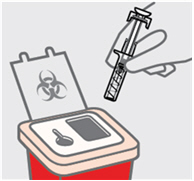
Dispose of your prefilled syringe
Put your used Simponi prefilled syringe in an FDA-cleared sharps disposal container right away after use.
Do not throw away (dispose of) your Simponi prefilled syringe in your household trash.
Do not recycle your used sharps disposal container.
For more information, see "How should I dispose of the used prefilled syringe?"
Check injection site
There may be a small amount of blood or liquid at the injection site. Hold pressure over your skin with a cotton ball or gauze pad until any bleeding stops.
Do not rub the injection site.
If needed, cover injection site with a bandage.
 Need help?
Need help?
Call your healthcare provider to talk about any questions you may have. For additional assistance or to share your feedback call 800-JANSSEN (800-526-7736).
How should I dispose of the used prefilled syringe?
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
made of a heavy-duty plasticcan be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come outupright and stable during useleak-resistantproperly labeled to warn of hazardous waste inside the containerWhen your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: www.fda.gov/safesharpsdisposal
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Janssen Biotech, Inc. Horsham, PA 19044
US License No. 1864
Revised: May 2018
janssen
PRINCIPAL DISPLAY PANEL - 50 mg/0.5 mL Syringe CartonNDC 57894-070-01
FOR SUBCUTANEOUS INJECTION
Sterile solution in a single dose
prefilled syringe. Discard unused portion.
See package insert for dosing information.
No U.S. standard of potency.
This Product Contains
Dry Natural Rubber.
Rx only.
Simponi®
golimumab
50 mg / 0.5 mL
One single-dose prefilled syringe
NDC 57894-070-02
FOR SUBCUTANEOUS INJECTION
Sterile solution in a single-dose
SmartJect® autoinjector.
Discard unused portion.
See package insert for dosing information.
No U.S. standard of potency.
This Product Contains
Dry Natural Rubber.
Rx only.
Simponi®
golimumab
50 mg / 0.5 mL
One single-dose SmartJect® autoinjector
NDC 57894-071-01
FOR SUBCUTANEOUS INJECTION
Sterile solution in a single-dose
prefilled syringe. Discard unused portion.
See package insert for dosing information.
No U.S. standard of potency.
This Product Contains
Dry Natural Rubber.
Rx only.
Simponi®
golimumab
100 mg/mL
One single-dose prefilled syringe
NDC 57894-071-02
FOR SUBCUTANEOUS INJECTION
Sterile solution in a single-dose
SmartJect® autoinjector.
Discard unused portion.
See package insert for dosing information.
No U.S. standard of potency.
This Product Contains
Dry Natural Rubber.
Rx only.
Simponi®
golimumab
100 mg/mL
One single-dose SmartJect® autoinjector

|
Simponi golimumab injection, solution |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
Simponi golimumab injection, solution |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Labeler - Janssen Biotech, Inc. (099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Baxter Pharmaceutical Solutions | 604719430 | MANUFACTURE(57894-070, 57894-071), ANALYSIS(57894-070, 57894-071) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Biologics, B.V. | 409612918 | API MANUFACTURE(57894-070, 57894-071), ANALYSIS(57894-070, 57894-071) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cilag AG | 483237103 | ANALYSIS(57894-070, 57894-071), PACK(57894-070, 57894-071), LABEL(57894-070, 57894-071) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Sciences Ireland UC | 986030167 | API MANUFACTURE(57894-070, 57894-071), ANALYSIS(57894-070, 57894-071) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Anderson Brecon | 053217022 | PACK(57894-070, 57894-071), LABEL(57894-070, 57894-071) | |